A kind of preparation method of indoledione compound
A technology for indole dione and compound, which is applied in the field of pharmaceutical and organic chemistry synthesis, can solve the problems of complicated operation, influence on large-scale production and wide application, and high reaction temperature, achieves simple operation, good application prospect, and is suitable for large-scale production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
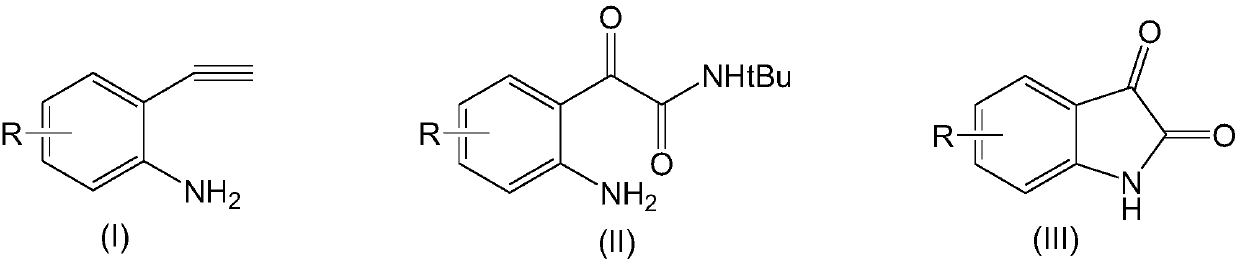
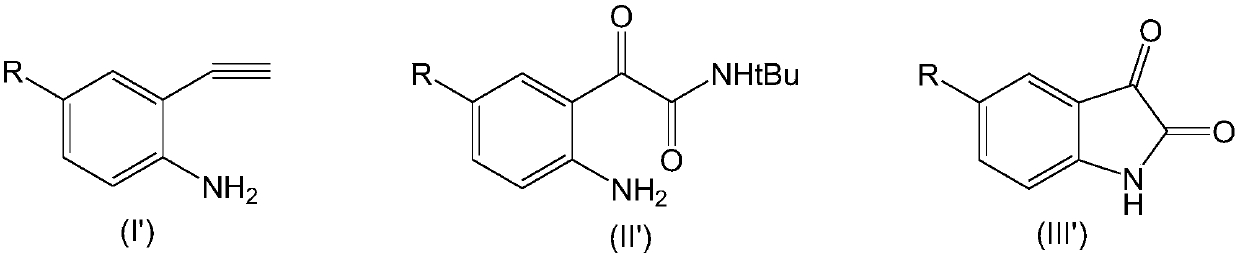
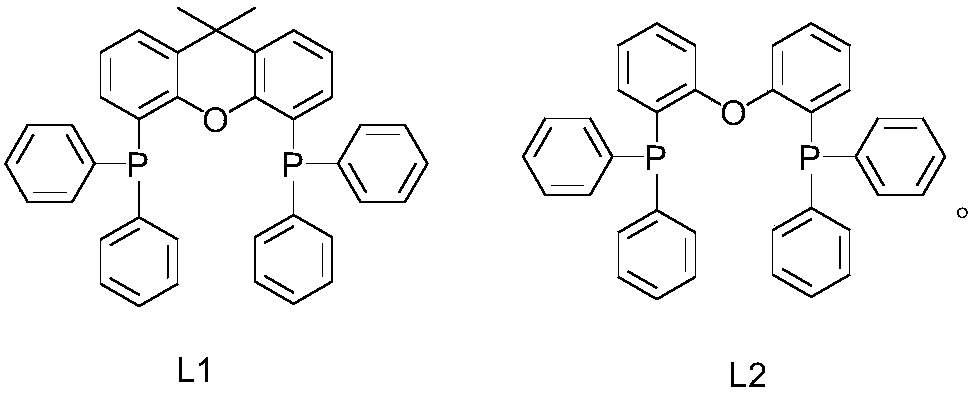
[0035] Add 20ml dimethyl sulfoxide and 1mmol compound of formula (I-1), 1.5mmol tert-butylamine, 0.1mmol rhodium triphenylphosphinecarbonyl acetylacetonate, 0.1mmol ligand L1, 2mmol bis(trifluoroacetic acid) in the reactor Iodobenzene and 1 mmol triethylamine were reacted with stirring at 60°C for 8 hours. After the reaction was completed, the reaction mixture was concentrated and evaporated to dryness, the residue was dissolved in chloroform, washed with water, organically dried over anhydrous sodium sulfate, filtered and evaporated to remove the solvent, and the resulting residue was separated by silica gel column chromatography , the above-mentioned compound of formula (II-1) was obtained with a yield of 96.2%. 1H NMR (CDCl 3 ,400MHz): δ9.58(s,1H,Ar-H); 8.12(d,8Hz,1H,Ar-H); 7.03(br s,2H,NH 2 ); 6.79(br s,1H,CONH); 6.70(d,8Hz,1H,Ar-H); 1.40(s,9H,C(CH 3 ) 3 ).
[0036] Dissolve the obtained compound of formula (II-1) in 20 ml of tetrahydrofuran, add aqueous h...
Embodiment 2
[0038]
[0039] Add 20ml dimethyl sulfoxide and 1mmol compound of formula (I-2), 1.5mmol tert-butylamine, 0.15mmol rhodium triphenylphosphinecarbonyl acetylacetonate, 0.2mmol ligand L1, 3mmol bis(trifluoroacetic acid) in the reactor Iodobenzene and 1.5 mmol triethylamine were stirred and reacted at 50°C for 8 hours. After the reaction was completed, the reaction mixture was concentrated and evaporated to dryness, the residue was dissolved in chloroform, washed with water, organically dried over anhydrous sodium sulfate, filtered and evaporated to remove the solvent, and the resulting residue was separated by silica gel column chromatography , the compound of formula (II-2) was obtained with a yield of 95.3%. 1 H NMR (CDCl 3,400MHz): δ8.44(s,1H,Ar-H); 7.18(d,8Hz,1H,Ar-H); 6.71(br s,1H,CONH); 6.59(d,8Hz,1H,Ar- H); 6.23 (brs,2H,NH 2 ); 1.42(s,9H,C(CH 3 ) 3 ).
[0040] Dissolve the obtained compound of formula (II-2) in 20ml of tetrahydrofuran, add aqueous hydrochloric ac...
Embodiment 3
[0042]
[0043] In the reactor, add 20ml dimethyl sulfoxide and 1mmol compound of formula (I-3), 1.5mmol tert-butylamine, 0.1mmol rhodium triphenylphosphinecarbonyl acetylacetonate, 0.1mmol ligand L2, 2mmol bis(trifluoroacetic acid) Iodobenzene and 1.5 mmol triethylamine were stirred and reacted at 60°C for 8 hours. After the reaction was completed, the reaction mixture was concentrated and evaporated to dryness, the residue was dissolved in chloroform, washed with water, organically dried over anhydrous sodium sulfate, filtered and evaporated to remove the solvent, and the resulting residue was separated by silica gel column chromatography , the compound of formula (II-3) was obtained with a yield of 93.8%. 1 H NMR (CDCl 3 ,400MHz):δ8.14(s,1H,Ar-H); 7.12(d,8Hz,1H,Ar-H); 6.61(br s,1H,CONH); 6.56(d,8Hz,1H,Ar- H); 6.08 (brs,2H,NH 2 ); 2.19(s,3H,CH 3 ); 1.41(s,9H,C(CH 3 ) 3 ).
[0044] Dissolve the obtained compound of formula (II-2) in 20ml of tetrahydrofuran, add aque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com