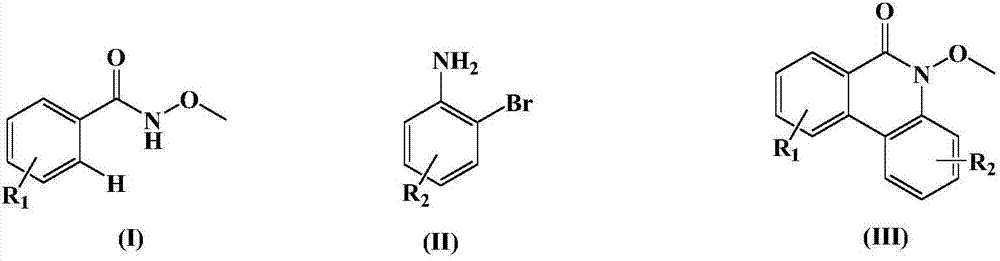
Synthesis method of medical intermediate phenanthridone compound
A synthesis method and compound technology, applied in the field of synthesis of phenanthridone compounds, can solve the problems of high cost, complicated operation, environmental pollution, etc., and achieve the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
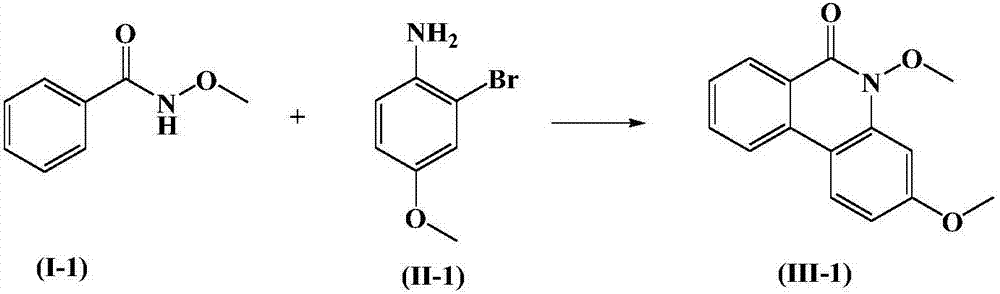
Embodiment 1
[0024]
[0025] Under the protection of an inert gas, add 100ml of mixed organic solvent of acetic acid and acetonitrile with a volume ratio of 2:1 in the reactor, add 10mmol of the above formula (I-1) compound, 12mmol of the above formula (II-1) compound, 1 mmol catalyst copper trifluoromethanesulfonate, 1 mmol cocatalyst, 20 mmol bis(trifluoroacetic acid) iodobenzene, and then add 15 mmol isoamyl nitrite to the reaction solution. While stirring, the temperature was raised to 50° C., and the reaction was stirred at this temperature for 8 hours. After the reaction was completed, all volatiles were removed in vacuo, the product was extracted with ethyl acetate, the solvent was removed from the organic phase, and the resulting residue was separated by 300-400 mesh silica gel column chromatography, and the elution solvent was petroleum ether and acetic acid at a volume ratio of 4:1. The mixture of ethyl esters was used to obtain the compound of formula (III-1) with a yield of ...
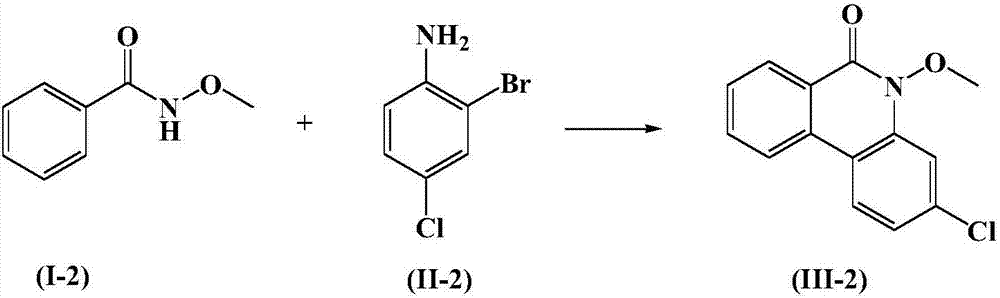
Embodiment 2
[0027]
[0028] Under the protection of an inert gas, add 10ml of a mixed organic solvent of acetic acid and acetonitrile with a volume ratio of 2:1 in the reactor, and add 1mmol of the above formula (I-2) compound and 1.2mmol of the above formula (II-2) compound to it successively , 0.1 mmol catalyst copper trifluoromethanesulfonate, 0.1 mmol cocatalyst, 2 mmol bis(trifluoroacetic acid) iodobenzene, and then add 1.5 mmol isoamyl nitrite to the reaction solution. While stirring, the temperature was raised to 50° C., and the reaction was stirred at this temperature for 8 hours. After the reaction was completed, all volatiles were removed in vacuo, the product was extracted with ethyl acetate, the solvent was removed from the organic phase, and the resulting residue was separated by 300-400 mesh silica gel column chromatography, and the elution solvent was petroleum ether and acetic acid at a volume ratio of 4:1. The mixture of ethyl esters can obtain the compound of formula ...
Embodiment 3
[0030]
[0031] Under the protection of an inert gas, add 10ml of a mixed organic solvent of acetic acid and acetonitrile with a volume ratio of 2:1 in the reactor, and add 1mmol of the above formula (I-3) compound and 1.2mmol of the above formula (II-3) compound to it successively , 0.12mmol catalyst copper trifluoromethanesulfonate, 0.12mmol cocatalyst, 2mmol bis(trifluoroacetic acid) iodobenzene, and then add 1.5mmol isoamyl nitrite in the reaction solution. While stirring, the temperature was raised to 50° C., and the reaction was stirred at this temperature for 8 hours. After the reaction was completed, all volatiles were removed in vacuo, the product was extracted with ethyl acetate, the solvent was removed from the organic phase, and the resulting residue was separated by 300-400 mesh silica gel column chromatography, and the elution solvent was petroleum ether and acetic acid at a volume ratio of 4:1. The mixture of ethyl esters was used to obtain the compound of fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com