Selective TNFR1 antagonist peptide SN10 and application thereof in rheumatoid arthritis
A rheumatoid, PEG-SN10 technology, applied in the field of biomedicine, can solve the problems of difficult to achieve effective therapeutic effect, small relative molecular mass, short plasma half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Synthesis of Qinghuan sea snake selective TNFR1 antagonistic peptide Hydrostatin-SN10
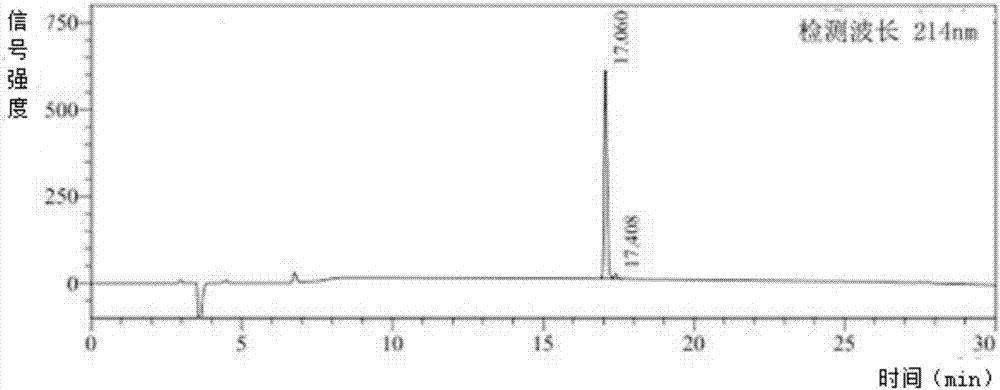
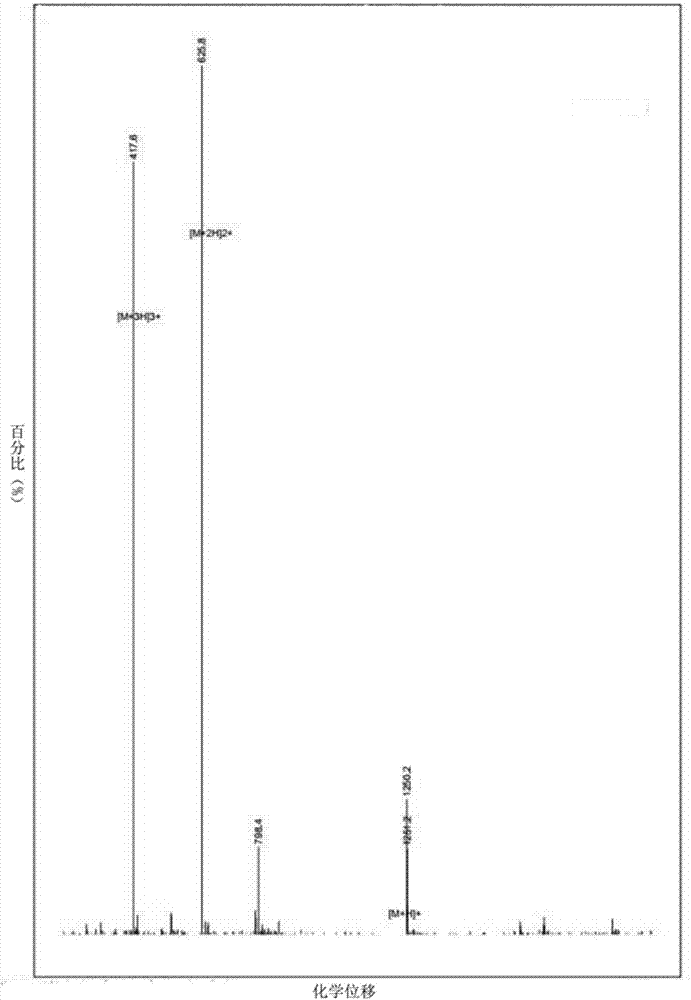
[0042] Hydrostatin-SN10 was synthesized by solid-phase peptide synthesis technology, and analyzed by HPLC ( figure 1 ) and MS ( figure 2 ) to analyze its purity and molecular weight, the result shows that its purity>97%, molecular weight is 1250.29g / mol.
Embodiment 2
[0043] Example 2: BIAcore analysis of the binding ability of Hydrostatin-SN10 to TNFR1.
[0044] 1. The running buffer flows through the channel set in the CM-5 sensor chip at a flow rate of 10 μl / min until it reaches the baseline level.
[0045] 2. Use the buffer recommended by the instrument to activate the surface reactive groups on each channel of the chip.
[0046] 3. Dissolve the freeze-dried powder of TNFR1 and TNFR2 with EP buffer, inject samples at a certain concentration to coat the surface of the chip, and then seal the chip with 1mol / L ethanolamine. Before determining the kinetic curve, the regeneration conditions should be tested to select the appropriate regeneration conditions.
[0047] 4. When the running buffer runs to a stable baseline, inject a series of peptides at a concentration, repeat the injection of a peptide at an intermediate concentration, and record the response value of each concentration.
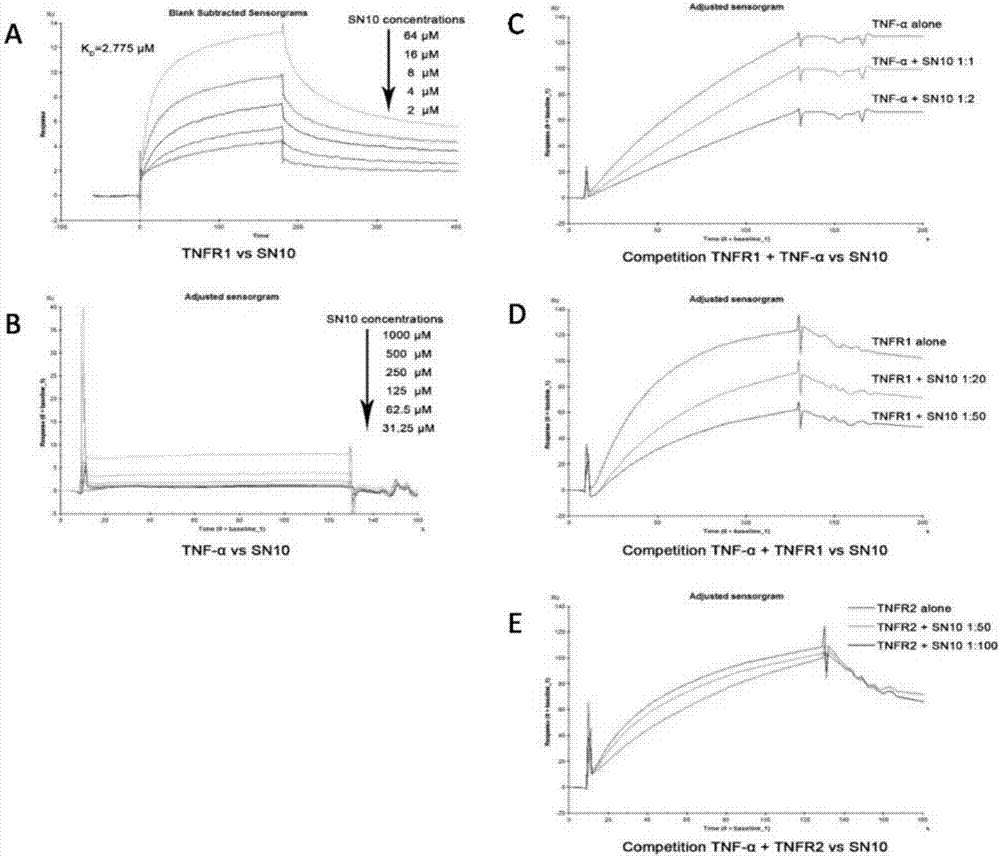
[0048] Such as image 3 As shown, Hydrostatin-SN10 c...
Embodiment 3
[0049] Example 3: MST analysis of the binding ability of Hydrostatin-SN10 and TNFR1.
[0050] 1. Interaction between Hydrostatin-SN10 and TNF-α, TNFR1, TNFR2:
[0051] Prepare a series of gradient concentrations of Hydrostatin-SN10 at a dilution ratio of 1:1, mix equal volumes of fluorescently labeled TNF-α / TNFR1 / TNFR2 200nM with Hydrostatin-SN10 and incubate for 30 minutes in the dark, then use a capillary pipette to draw an appropriate amount of sample on the machine Detect, observe the time trajectory of the relative fluorescence value and the dose-response curve of thermophoresis, and calculate the affinity K by fitting the software NTAffinityAnalysis v2.0.2 D value to determine whether there is a specific binding trend.
[0052] 2. Competitive inhibitory effect of Hydrostatin-SN10 on the combination of TNF-α and TNFR1 / TNFR2:
[0053] Prepare a series of gradient concentrations of TNF-α at a dilution ratio of 1:1, uniformly mix equal volumes of fluorescently labeled TNFR...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com