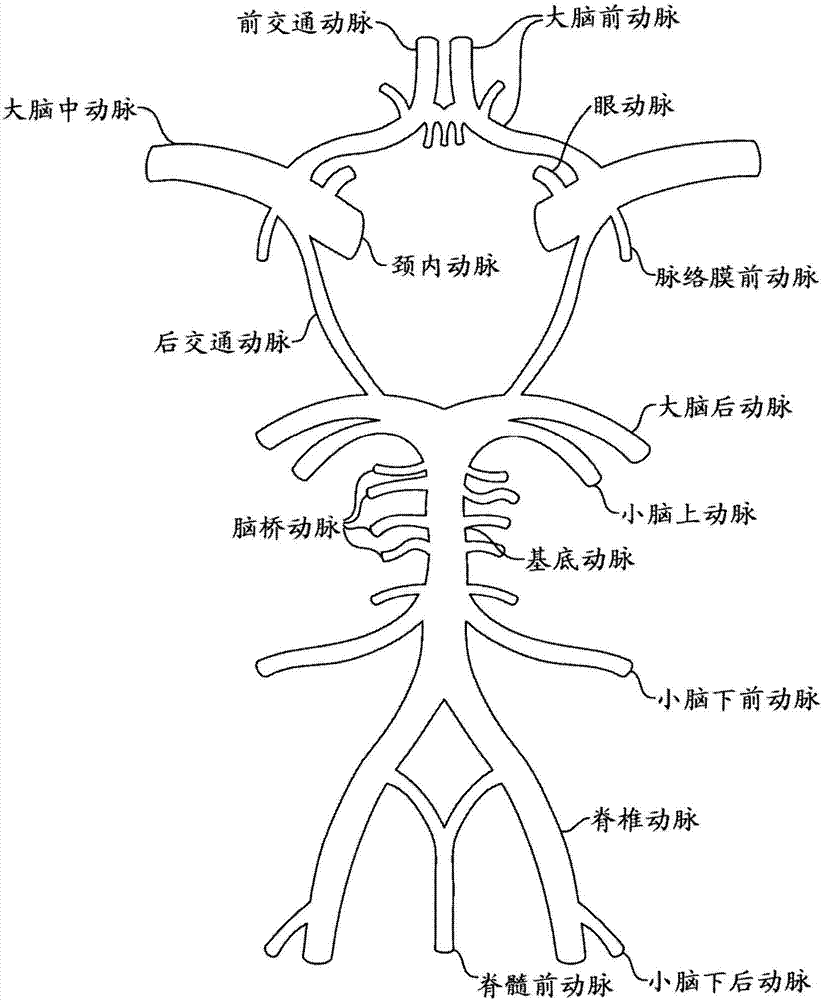
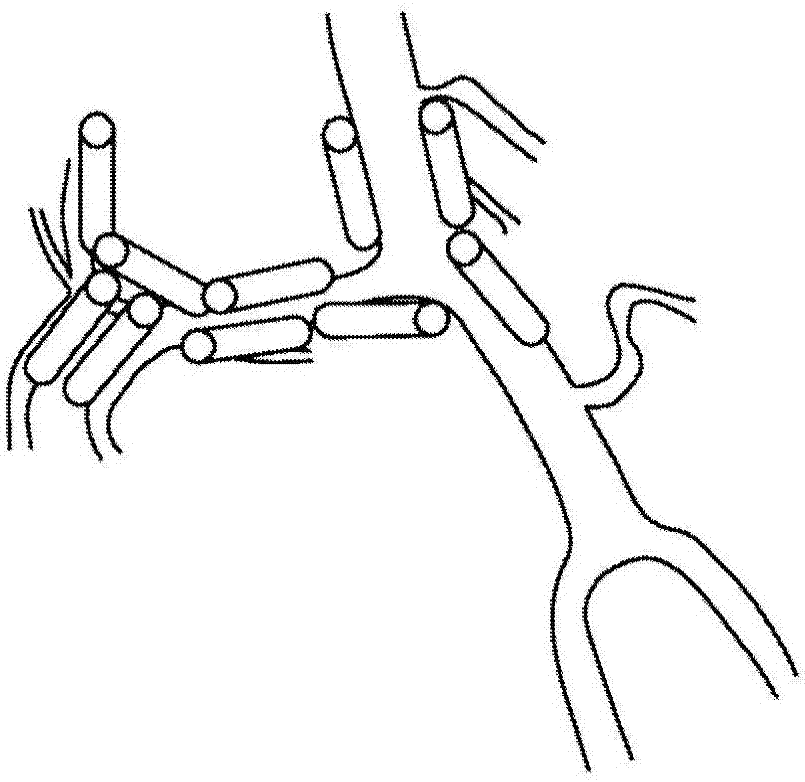
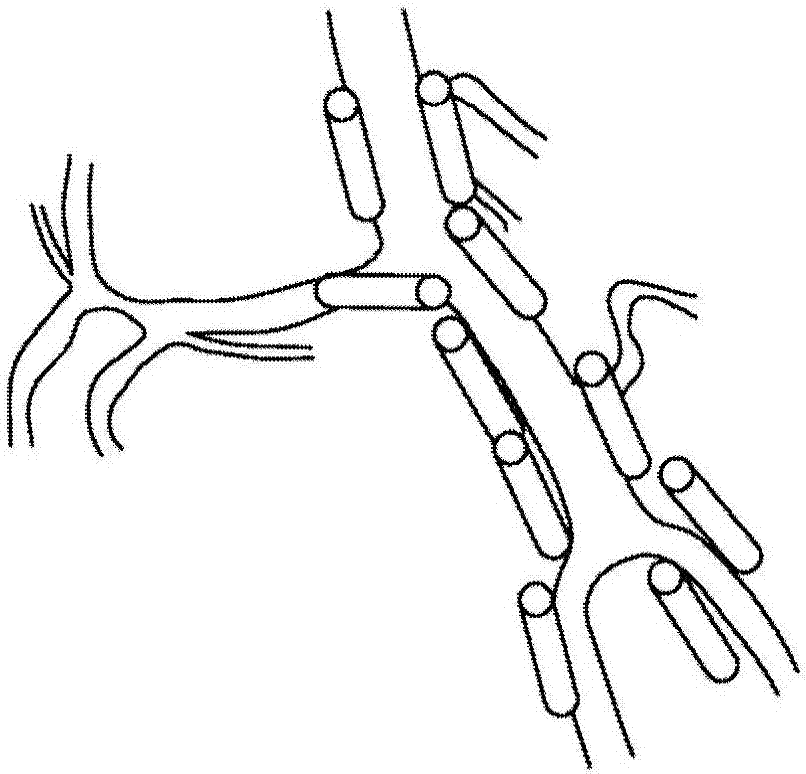
Compositions and methods for improving prognosis of a human with subarachnoid hemorrhage
A subarachnoid space and composition technology, which can be used in drug combinations, active ingredients of heterocyclic compounds, blood diseases, etc., can solve problems such as expensive, time-consuming, and hindering systemic doses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0481] Example 1. Pilot Study 1 - Effect of Nimodipine Formulation on Cerebral Vasospasm in Canine Model of Subarachnoid Hemorrhage (SAH)
[0482] Materials and Methods
[0483] preparation
[0484] A test formulation of a microparticulate nimodipine formulation with uniform particle size distribution was prepared by combining a polymer solution (eg, a 50-50 glycolide-lactide blend) with a solvent in the presence of nimodipine. The mixture is added to a surfactant-containing aqueous solution to form an emulsion, and the solvent is separated off to produce a flowable microparticle nimodipine formulation. The initial drug loading was 65%, ie, 65% nimodipine and 35% polymer. The average particle size is about 52μ.
[0485] The microparticulate nimodipine formulation is combined with a pharmaceutical carrier to form the pharmaceutical composition of the present invention. When the device for delivery is a surgical injection device and the site of delivery is a cerebral artery ...
Embodiment 2
[0539] Example 2. Study 2 - Effect of nimodipine formulations on cerebral vasospasm in a canine model of subarachnoid hemorrhage (SAH)
[0540] Materials and Methods
[0541] preparation
[0542] A test formulation comprising a microparticulate nimodipine formulation with a uniform particle size distribution was prepared by combining a polymer solution (eg, a 50-50 glycolide-lactide blend) with a solvent in the presence of nimodipine. The mixture is added to a surfactant-containing aqueous solution to form an emulsion, and the solvent is separated off to produce a flowable microparticle nimodipine formulation. The initial drug loading was 65%, ie, 65% nimodipine and 35% polymer. The average particle size is about 52μ.
[0543] The microparticulate nimodipine formulation is combined with a pharmaceutical carrier to form the pharmaceutical composition of the present invention. When the device used for delivery is a surgical injection device and the site of delivery is in clo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com