Gip and glp-1 co-agonist compounds
A compound, conjugation technology, applied in the field of medicine, which can solve problems such as low immunogenicity potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
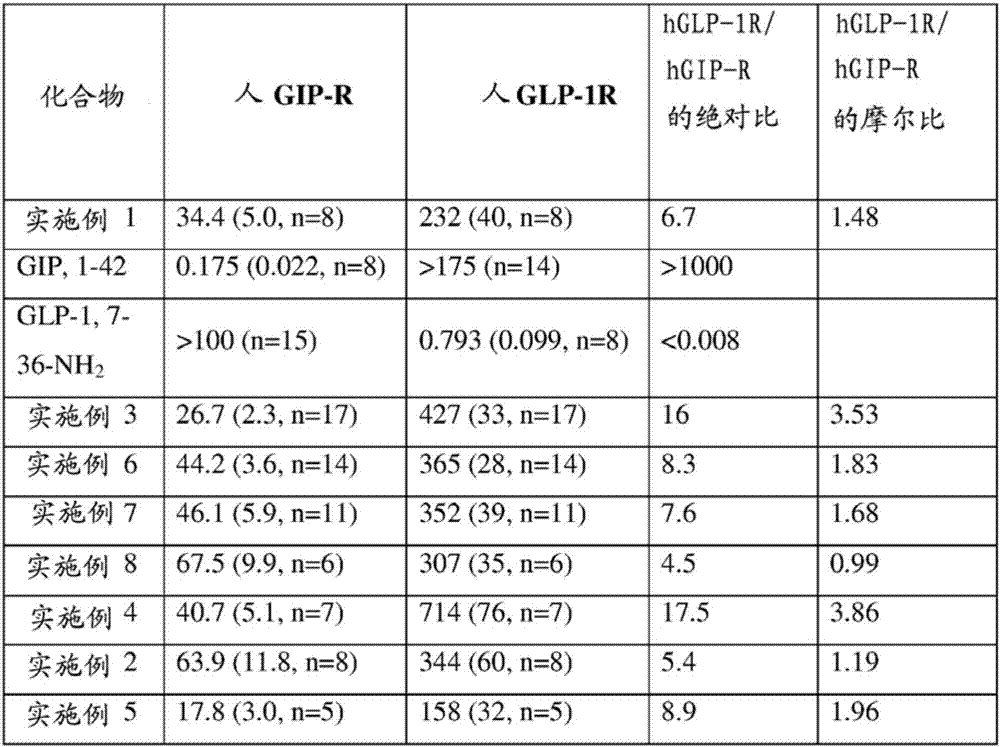
Embodiment 1
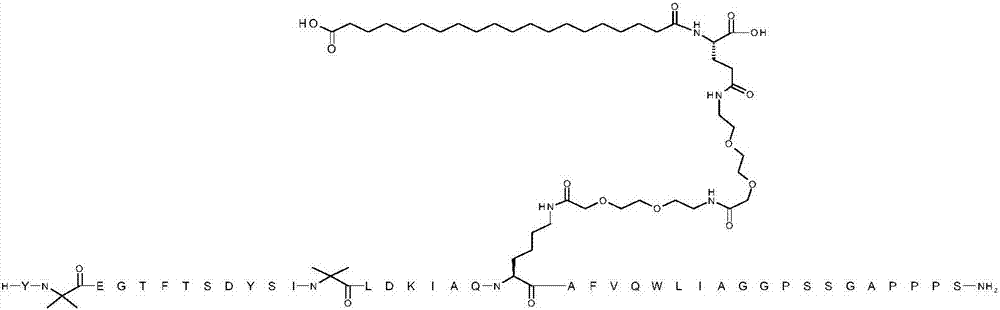
[0065] YX 1 EGTFTSDYSIX 2 LDKIAQKAFVQWLIAGGPSSGAPPPS
[0066] where X 1 is Aib;X 2 is Aib; K at position 20 is replaced by ([2-(2-amino-ethoxy)-ethoxy]-acetyl) 2 -(γGlu) 1 -CO-(CH 2 ) 18 -CO 2 Chemically modified by H conjugation to the ε-amino group of the K side chain; and the C-terminal amino acid is amidated to a C-terminal primary amide (SEQ ID NO:3)
[0067] Trifluoroacetate
[0068]
[0069] The structure above contains the standard one-letter amino acid code except for residues Aib2, Aib13 and K20, where the structure of the amino acid residues Aib2, Aib13 and K20 has been expanded.
[0070] The peptide of SEQ ID NO: 3 according to the present invention was generated by solid-phase peptide synthesis using the Fmoc / t-Bu strategy on a Symphony automated peptide synthesizer (PTIProtein Technologies Inc.), starting from RAPP AM-Rink amide resin, where 6 equivalents of amino acids activated with diisopropylcarbodiimide (DIC) and hydroxybenzotriazole (HOBt) (1:1...
Embodiment 2
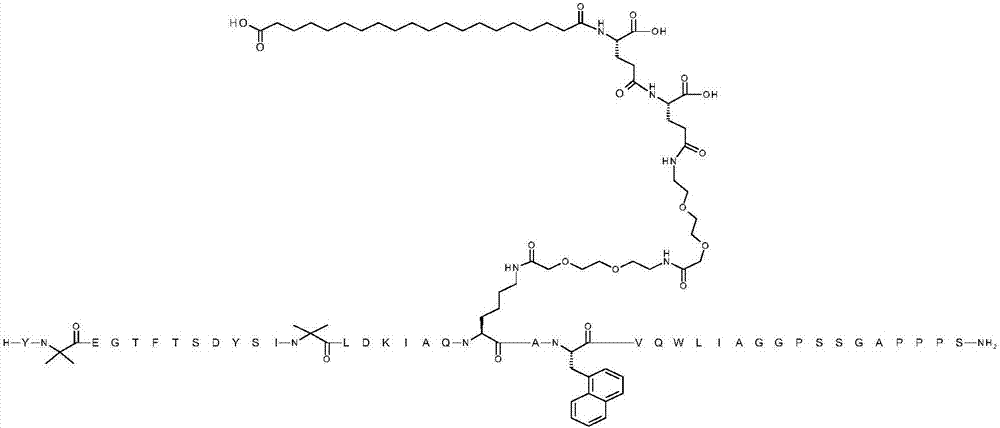
[0076] TX 1 EGTFTSDYSIX 2 LDKIAQKAX 3 VQWLIAGGPSSGAPPPS
[0077] where X 1 is Aib;X 2 is Aib; K at position 20 is replaced by ([2-(2-amino-ethoxy)-ethoxy]-acetyl) 2 -(γGlu) 2 -CO-(CH 2 ) 18 -CO 2 H is chemically modified by conjugation to the ε-amino group of the K side chain; X 3 is 1-Nal; and the C-terminal amino acid is amidated to the C-terminal primary amide (SEQ ID NO:4)
[0078] Trifluoroacetate
[0079]
[0080] The above structure contains the standard one-letter amino acid code except for residues Aib2, Aib13, K20 and 1-Nal22, for which the structure of these amino acid residues Aib2, Aib13, K20 and 1-Nal22 has been expanded.
[0081] The peptide of SEQ ID NO: 4 according to the present invention was synthesized similarly to that described in Example 1 above. The following conditions were used for the coupling of Fmoc-1Nal-OH at position 22: 25°C, Fmoc-1Nal-OH (6 equiv), PyBOP (6 equiv) and DIEA (12 equiv) in DMF for 4 hours.
Embodiment 3
[0083] YX 1 EGTFTSDYSIX 2 LDKIAQKAFVQWLIAGGPSSGAPPPS
[0084] where X 1 is Aib;X 2 is Aib; K at position 20 is replaced by ([2-(2-amino-ethoxy)-ethoxy]-acetyl) 2 -(γGlu) 1 -CO-(CH 2 ) 16 -CO 2 Chemically modified by H conjugation to the ε-amino group of the K side chain; and the C-terminal amino acid is amidated to a C-terminal primary amide (SEQ ID NO:5)
[0085] Trifluoroacetate
[0086] Similar to what was described in Example 1 above, the compound of SEQ ID NO: 5 according to the present invention was synthesized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com