A kind of b-type influenza virus broad-spectrum neutralizing antibody, its preparation method and application
An influenza virus and antibody technology, applied in chemical instruments and methods, botanical equipment and methods, biochemical equipment and methods, etc., can solve the problems of being unable to prevent bird flu and swine flu, and not keeping up with the speed of seasonal influenza virus mutation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
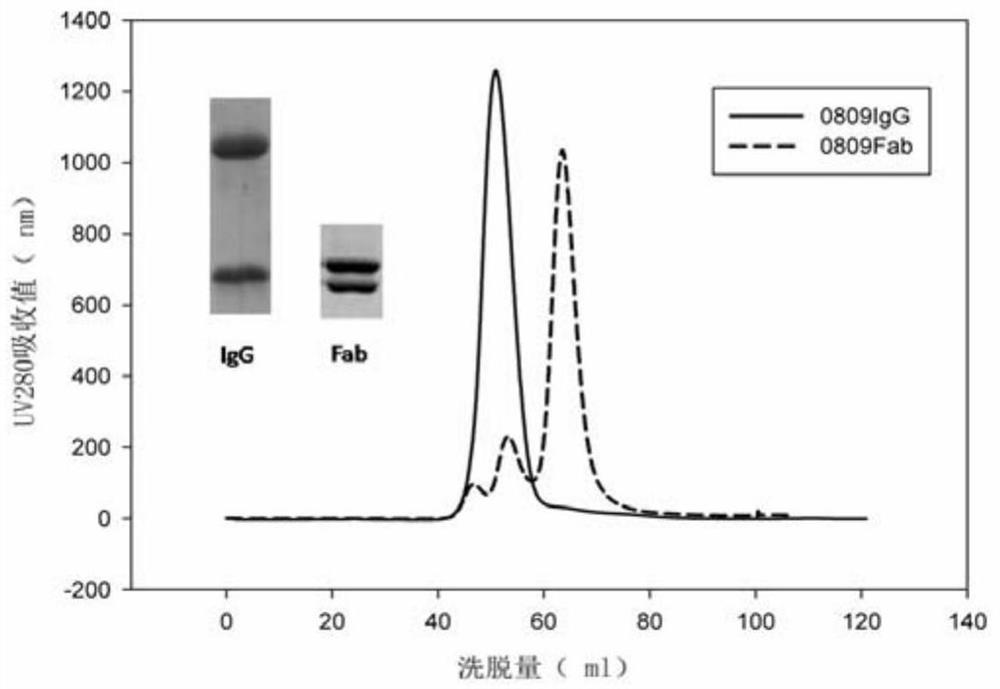
[0068] Example 1 Preparation of Antibody
[0069] The preparation method of broad-spectrum neutralizing antibody of type B influenza virus comprises the following steps:
[0070] (1) PBMCs in peripheral blood were collected on day 0 and day 7 after volunteers were inoculated with seasonal influenza vaccine, RNA was extracted, and cDNA was reverse transcribed;
[0071] (2) Amplify the hypervariable region sequences of the heavy chain and light chain, sequence the amplified target fragments using Miseq 2 X 300bp, and analyze the sequencing results;
[0072] (3) Using CDR abundance as the main parameter to select the high-frequency variable region sequences of immunized persons 7 days after immunization, and calculate the probability of natural pairing through the heavy chain and light chain pairing algorithm, and then select the high frequency occurrence of CDR1, CDR2 and CDR3 The VH and VL sequences plus their respective constant regions are synthesized;
[0073] The nucleoti...
Embodiment 2
[0080] Example 2 Antigen-antibody affinity test
[0081] Using surface plasmon resonance technology, the Fab (10 μg / mL) form of 0809 was fixed on the CM5 chip through amino coupling, and the fixed value reached 500RU. The mobile phase antigen protein influenza virus type B HA was diluted by 2 times (concentration range 1 μM-100 μM). Select the KINJECT mode for the determination of kinetic parameters, the flow rate of the machine is 30 μL / min, the injection time is 1-2 minutes, and the dissociation time is 2-6 minutes. Then, the affinity was calculated using BIA evaluation software, and Table 1 shows the affinity measurement results between 0809 Fab and various strains of influenza B virus HA. It can be seen from Table 1 that the HA binding and dissociation modes of 0809 and the strains of influenza B viruses Yamagata and Victoria are almost all fast binding and fast dissociation, with affinities of 4.11nM and 7.63nM, respectively.
[0082] Table 1. Affinity determination of ...
Embodiment 3
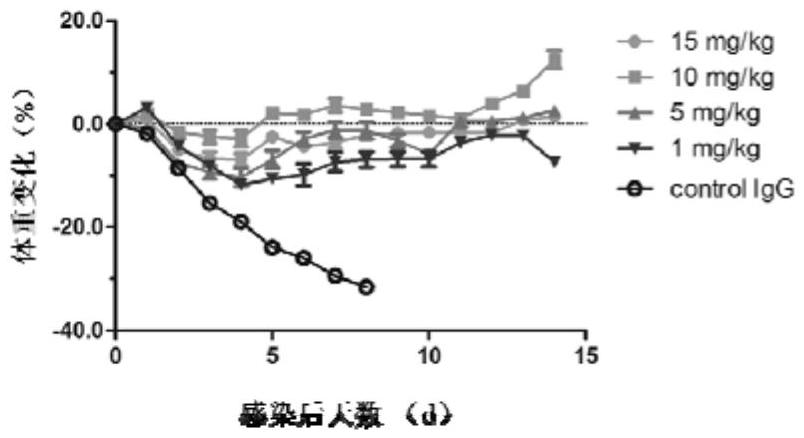
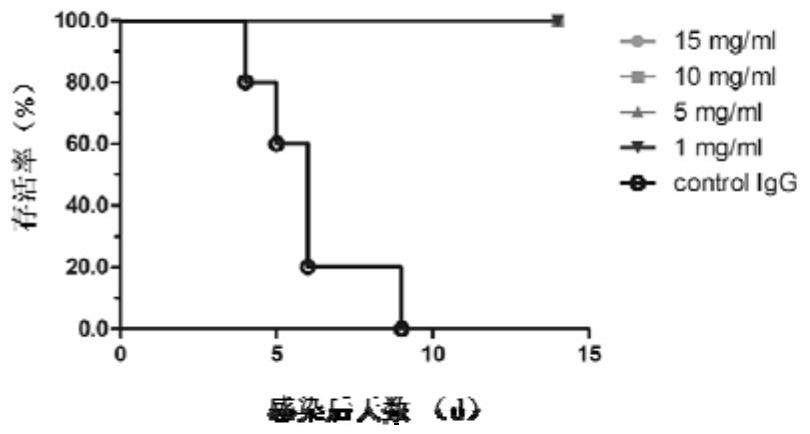
[0084] Example 3 Hemagglutination inhibition (HI) test and neutralization test of antibodies
[0085] Hemagglutination inhibition test: using the method of fixing the virus diluted antibody, the 0809 IgG antibody dilution was reacted with 4 hemagglutination units of influenza B virus at room temperature for 30 minutes, and then 1% chicken red blood cells of the same volume as the virus solution were added, Table 2 The hemagglutination inhibitory activity of 0809 IgG to various strains of influenza B viruses is shown. It was found that 0809 IgG is a hemagglutination-inhibiting antibody, which has high hemagglutination inhibitory activity against different strains of influenza B virus, and the HI titer is between 0.24μg / ml-4.7μg / ml.
[0086] Neutralization test: using the method of fixing virus diluted antibody, dilute 200TCID of the same volume of 0809IgG antibody dilution 50 Different influenza B virus strains were mixed together and left at room temperature for 1 hour. Then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com