A cancer diagnosis chip and its kit
A gastric cancer, 1.hsa-mir-092a-1 technology, applied in the field of medicine and biology, can solve the problems of insufficient sensitivity of detection and difficulty in early screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
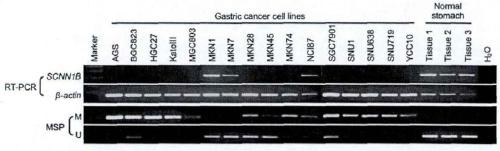
[0019] Example 1 Differential miR analysis in gastric cancer
[0020] 1. Determination of samples
[0021] This experiment selects a large-sample gastric cancer clinical cohort study from the Prince of Wales Hospital in Hong Kong (1998-2002, the average follow-up time is 40.8 months, the longest is 157.9 months), and each case is guaranteed to have more than 5 years of experience. Follow-up data and complete clinical information. This cohort study included 245 cases, all of whom were directly surgically resected without neoadjuvant therapy and were pathologically confirmed as primary gastric cancer. All gastric cancer case information and tissue samples were collected. After deliberation and approval by the Clinical Research Ethics Committee of the Chinese University of Hong Kong, and the informed consent of the patients and their families. Gastric cancer tissues and their paired distal non-tumor gastric tissues were collected during gastric cancer surgery. After collection, t...
Embodiment 2
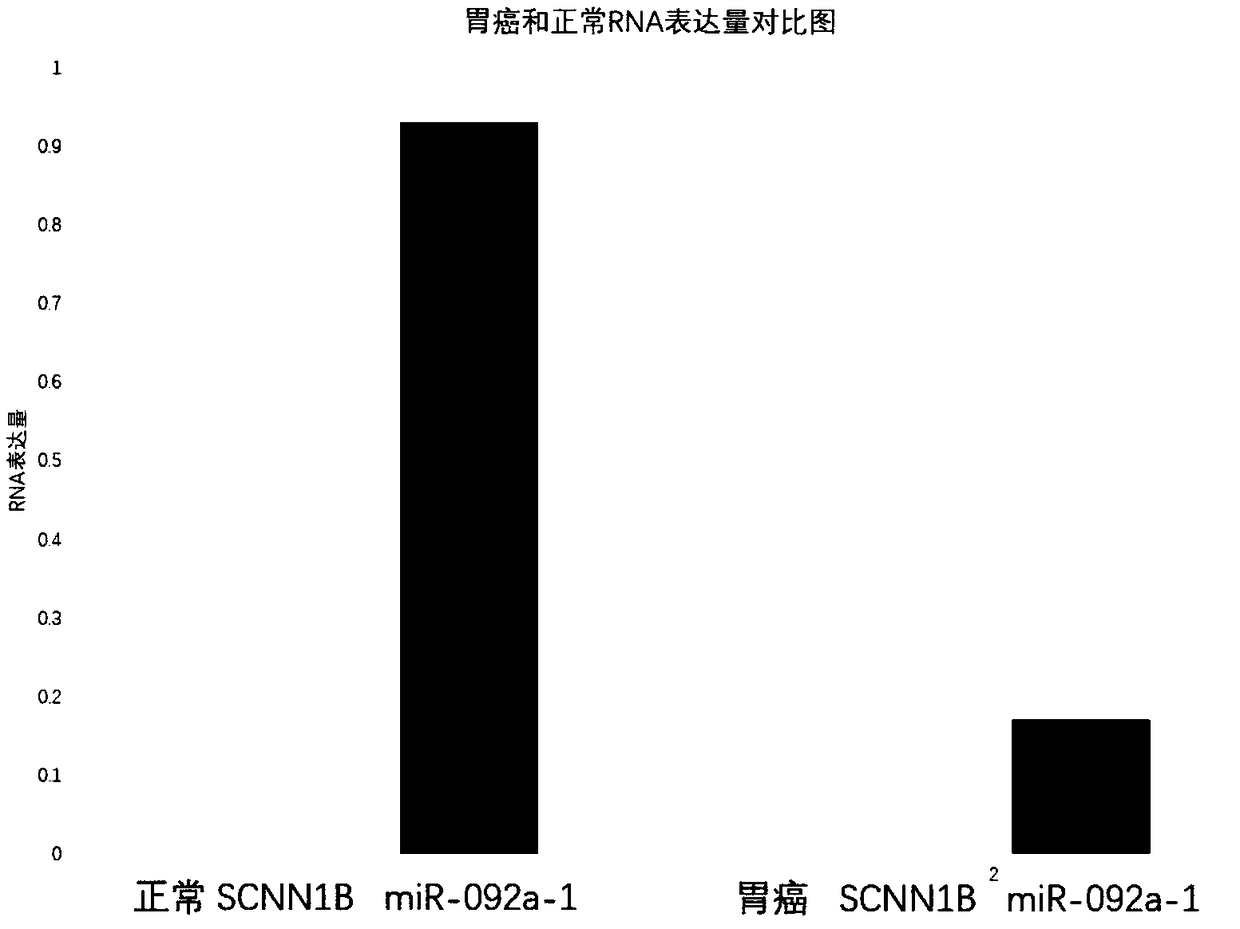
[0024] Example 2 Effect of Highly Expressed hsa-miR-092a-1 on Cancer Cells
[0025] Gastric cancer cell lines AGS and BGC823 were cultured; the culture medium used was high entanglement DMEM medium (Dulbecco'S modified Eagle's medium, Gibco-Invitrogen, Carlsbad, CA, USA). Cells were placed at 37°C, 5% CO 2 and cultured in a sterile incubator with saturated humidity. Under normal growth conditions, the culture medium was changed every 2-4 days, and the cells were passaged at 1:3 or 1:4 by 0.25% trypsin-EDTA solution (Trypsin-EDTA solution, Gibco-Invitrogen, Carlsbad, CA, USA).
[0026]Construction of an overexpression hsa-miR-092a-1 vector: the miR-092a-1 sequence and its flanking sequences were cloned into the pMD19-T vector, and sequenced by Shanghai Sunny Biotechnology Co., Ltd. The results showed that the sequence of miR-092a-1 was correct. The miR-092a-1 sequence and its flanking sequences confirmed by sequencing were subcloned and inserted between EcoRI / BamHI of the pC...
Embodiment 3
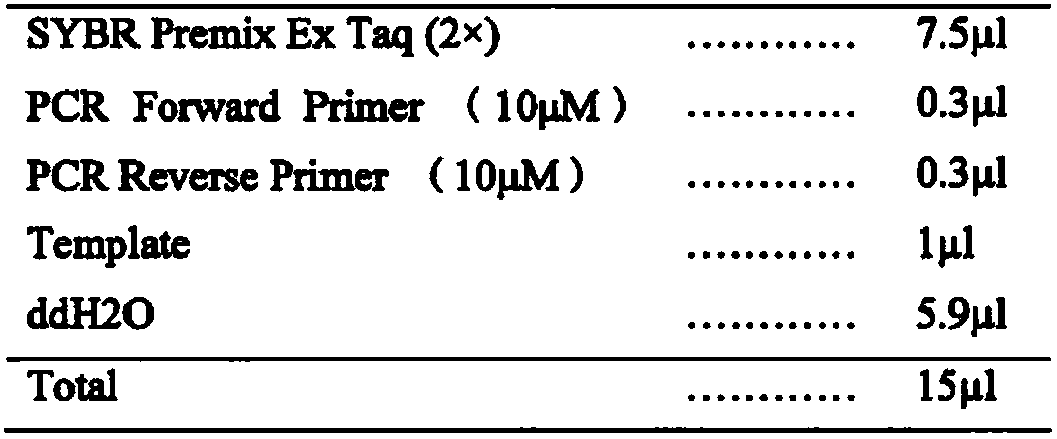
[0037] Example 3 Application of miR-092a-1 in detection of gastric cancer
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com