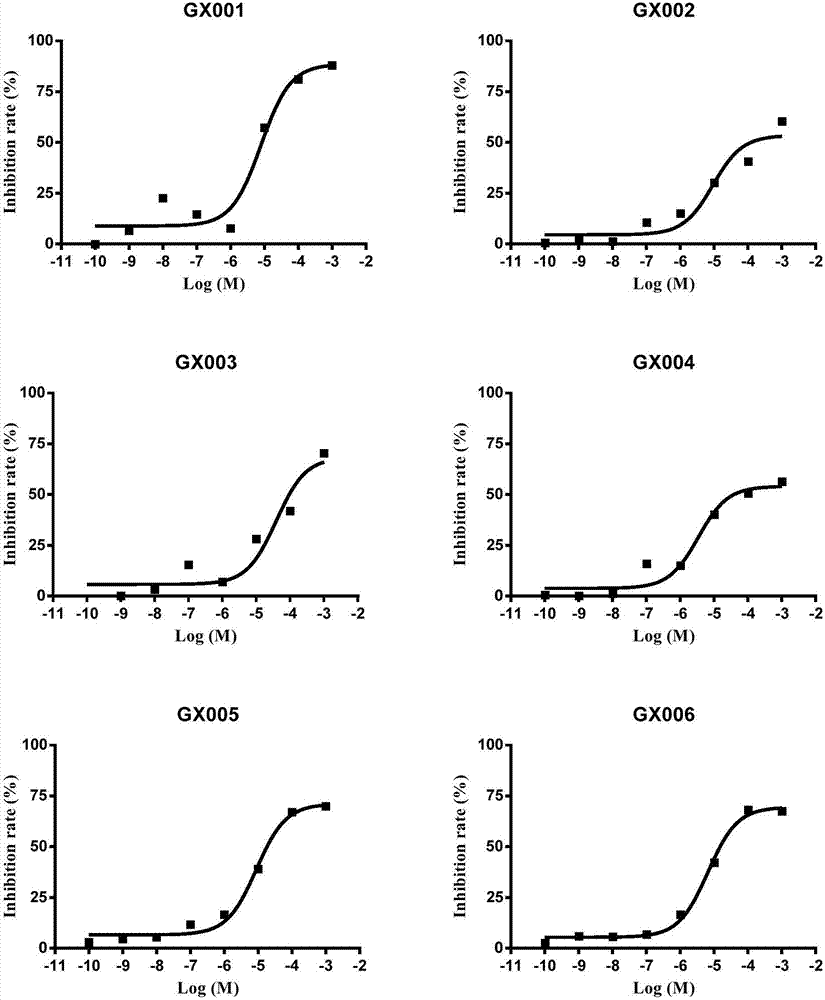
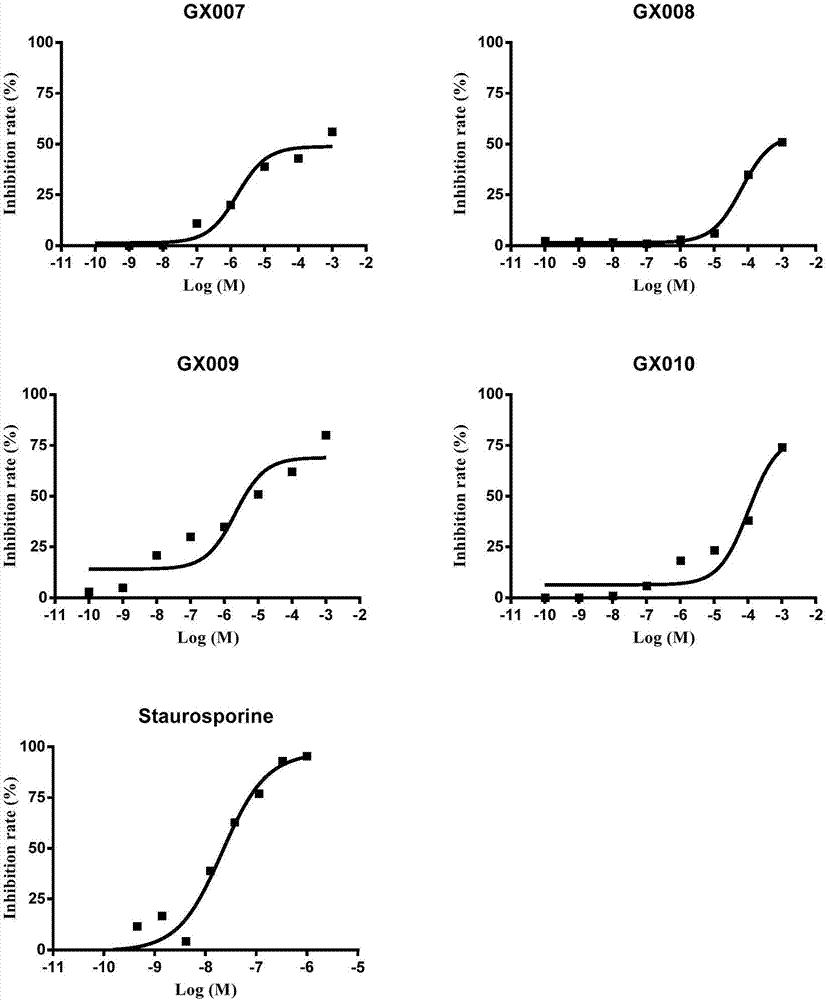
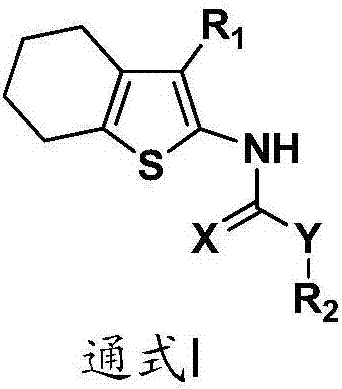
Tetrahydro benzothiophene derivative and application thereof to preparation of glycogen synthase kinase 3 beta inhibitor
A technology of benzothiophene and derivatives, applied in the field of medicine, can solve the problems of lack of effective drugs and treatment methods, achieve good GSK-3β inhibitory activity, high activity, and meet the effect of large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Synthesis of intermediate A (2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxamide)
[0045] Cyclohexanone (0.1mol) and 2-cyanoacetamide (0.1mol) were reacted in ethanol solution (30mL) to obtain compound 3, sulfur powder (0.1–0.11mol) was added to the reacted solution, and diethyl amine (10 mL), and keep the reaction temperature not exceeding 50 °C. After 3 hours of reaction, change to an ice-salt bath to make the reaction liquid crystallize. If there is no crystallization, pour the reaction liquid into 100 mL of water, filter with suction, and recrystallize the precipitated solid with ethanol to obtain the target compound, namely compound 4. Yield 61%.
[0046] Mp 190°C; 1 H-NMR (DMSO-d 6 ): d (ppm) 1.68 (m, 4H), 2.51 (m, 4H), 6.52 (bs, 2H), 6.89 (s, 2H); LRMS-EI: m / z calcd 196.1, found 196.0.
Embodiment 2
[0047] Example 2: Synthesis of GX001 (2-(3-cyclohexylurea)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxamide)
[0048] Dissolve compound 4 (0.80 mmol) in dry CH 2 Cl 2 (5 mL), add cyclohexyl isocyanate (12.8 mmol) and heat to reflux for 5d. After the reaction solution was cooled, 50% ethanol aqueous solution (5 mL) was added, and suction filtered to obtain the precipitated solid, which was recrystallized with ethanol to obtain the target product with a yield of 79%.
[0049] Mp 190.5-192.5°C; 1 H-NMR (DMSO-d 6 ):d(ppm)1.21(m,4H),1.49(m,6H),1.70(m,4H),2.52(m,4H),3.54(m,1H),6.97(bs,2H),7.53( s,1H),10.48(s,1H); ESI-MS m / z:322.1[M+H] + .
Embodiment 3
[0050] Example 3: Synthesis of GX002 (2-(3-cyclopentylurea)-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxamide)
[0051] Dissolve compound 4 (0.80 mmol) in dry CH 2 Cl 2 (5 mL), add cyclopentyl isocyanate (12.8 mmol) and heat to reflux for 5d. After the reaction solution was cooled, 50% ethanol aqueous solution (5 mL) was added, and suction filtered to obtain the precipitated solid, which was recrystallized with ethanol to obtain the target product with a yield of 61%.
[0052] Mp 200.3-202.5°C; 1 H-NMR (DMSO-d 6 ):d(ppm)1.50(m,4H),1.86(m,4H),1.70(m,4H),2.52(m,4H),3.61(m,1H),6.97(bs,2H),7.53( s,1H),10.48(s,1H); ESI-MS m / z:308.4[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com