Pharmaceutical products and stable liquid compositions of il-17 antibodies
A technology for liquid compositions and drug products, which can be used in drug combinations, antibodies, drug delivery, etc., and can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
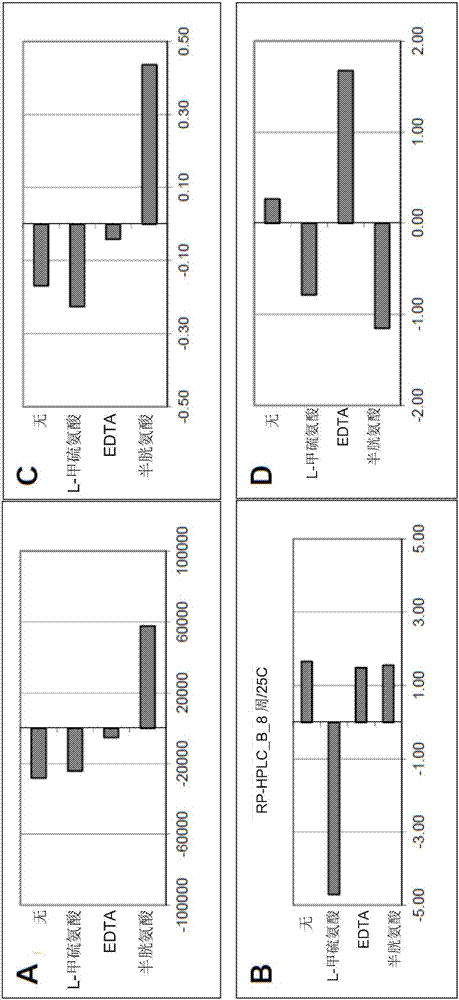
[0168] 1.1.1 Example 1: L-methionine
[0169] The effect of several antioxidant stabilizers on the stability of secukinumab was characterized using a large set of analytical techniques.
[0170] In earlier studies, a range of antioxidant stabilizers were evaluated, including tetrasodium EDTA, sodium ascorbate, cysteine, sodium bisulfate, and sodium citrate. Although none of these stabilizers sufficiently stabilized the molecule, tetrasodium EDTA and sodium citrate were observed on aggregated products measured by SEC compared to compositions without antioxidant stabilizers. Small stabilizing effect (data not shown).
[0171]In a further study, 10 mM concentrations of the stabilizers cysteine, tetrasodium EDTA, and L-methionine were evaluated and compared with no stabilizers using a secukinumab concentration of 150 mg / mL using the DoE method. Compositions were filled into PFS and subjected to 2-month stability studies under long-term (5°C), accelerated (25°C) and stress (40°C)...
Embodiment 2
[0183] 1.1.2 Example 2: Headspace Oxygen Content
[0184] 1.1.2.1 Primary Packaging - PFS:
[0185] The effect of headspace oxygen content on threo The influence of gold monoclonal antibody stability. Compositions were filled into 1 mL of PFS from each PFS supplier. The measured headspace oxygen content was between 13% and 15% (0.5 mL fill volume) or between 3-4% (0.5 mL fill volume) / 7-8% (1.0 mL fill volume), respectively. Store samples under long-term, accelerated and stress conditions for up to six months. Selected compositions were stored under long-term conditions for up to 24 months. Secukinumab stability was monitored by: SEC purity, RP-HPLC purity, CEX purity, CE-SDS purity (non-reducing), turbidity, color, free SH groups, biological activity, measured by photoblocking Insoluble particles and visible particles.
[0186] The effect of headspace oxygen content was observed by species and AP-SEC prior to passage through the RP-HPLC main peak under long-term, accel...
Embodiment 3
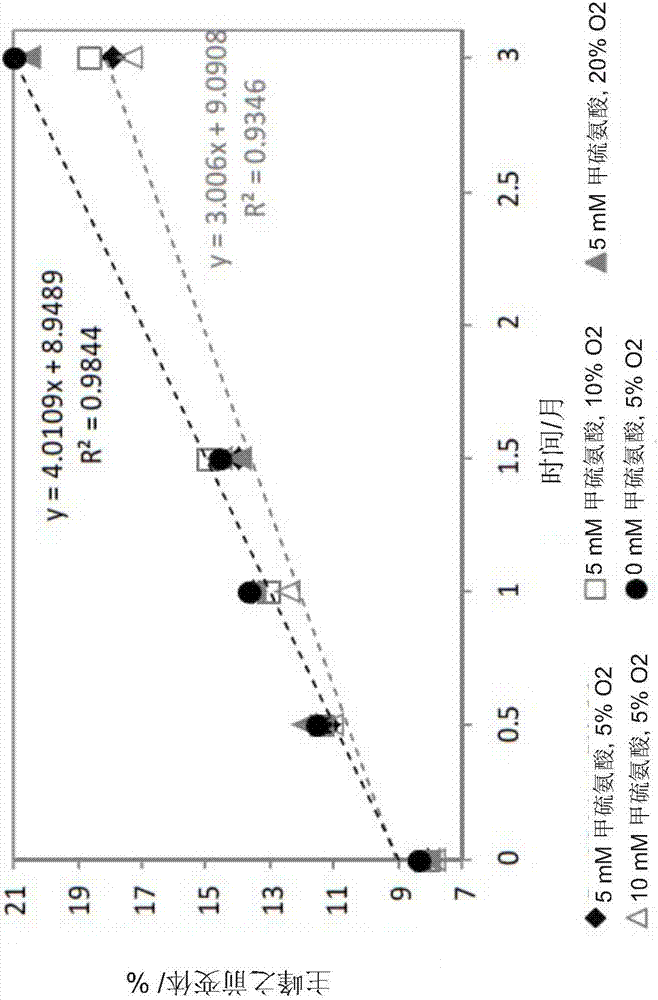
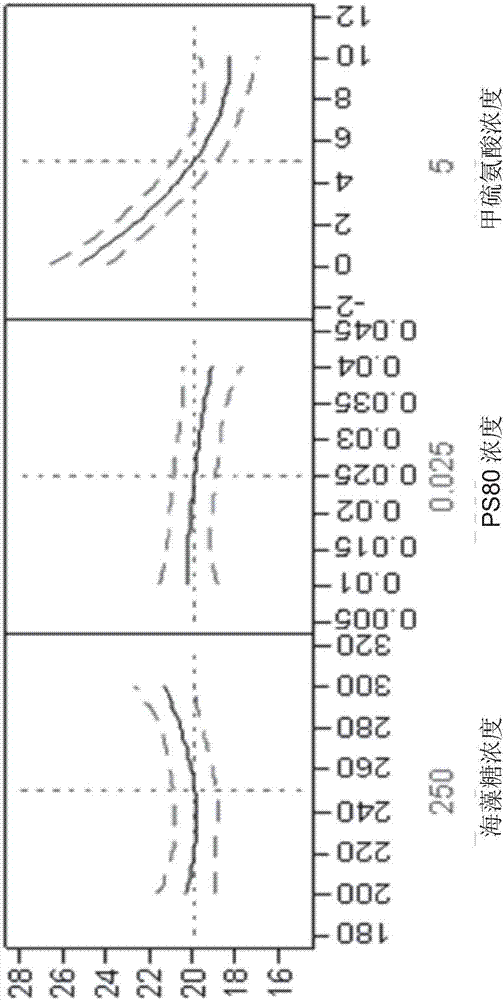
[0202] 1.1.3 Example 3: Interaction between L-methionine concentration and headspace oxygen content
[0203] Further studies evaluated the interaction between L-methionine concentration and headspace oxygen content. Compositions were prepared comprising L-methionine in the range of 2.5-7.5 mM and headspace oxygen content between 3 and 9%. Compositions were filled into PFS and stored for 6 months under long-term and accelerated conditions. The relevant secukinumab property attributes were monitored after 3 and 6 months of storage (SEC purity, RP-HPLC purity, CEX purity, free SH groups, biological activity, insoluble particulates and visible particles by light obscuration, turbidity and color of the solution). Figure 10 AP-SEC purity after 6 months storage at 25°C is shown as a function of L-methionine and headspace oxygen content. When analyzed using AP-SEC purity, no interactions were observed in the range tested.
[0204] In another study, the effect of reduced headspace...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com