Methods of treating lichen planus using interleukin-17 (IL-17) antagonists
A technology of lichen planus and antibodies, applied in the direction of antibody medical components, chemical instruments and methods, antibodies, etc., can solve problems such as side effects, intractability, and psychological discomfort
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
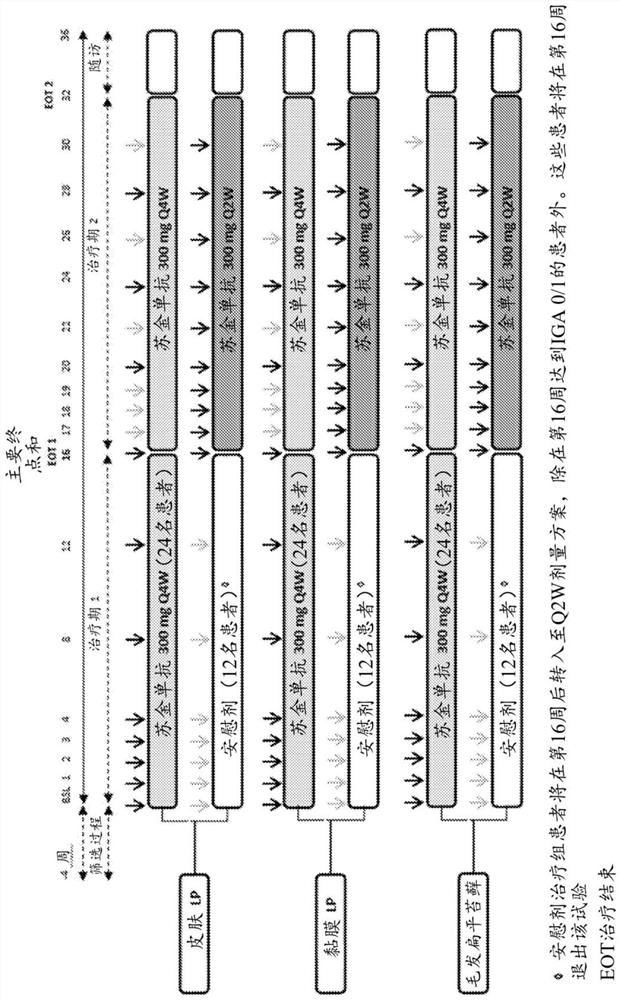
[0213] Example 1: Clinical Trial CAIN457S12201 - An Efficacy, Safety, and Tolerability of Secukinumab 300 mg Over 32 Weeks in Adult Patients with Biopsy-Confirmed Forms of Lichen Planus Inadequately Controlled with Topical Therapy Sexual Proof-of-Concept Study
[0214] Purpose and rationale of design:
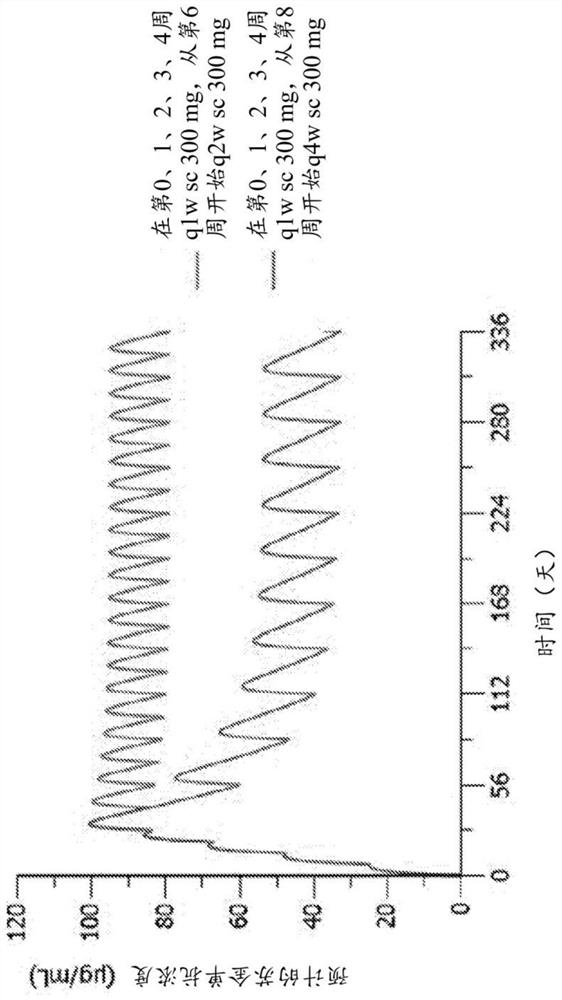
[0215] The purpose of this proof-of-concept study was to investigate the efficacy of secukinumab in the treatment of adult patients with biopsy-proven lichen planus inadequately controlled with topical therapy and to assess the safety and tolerability of treatment over 32 weeks sex. The double-blind, randomized, placebo-controlled design of this trial enables the evaluation of secukinumab 300 mg in two different dosing regimens and three selected subtypes of lichen planus (MLP, CLP, LPP) in an adequate and controlled setting ) efficacy and safety.
[0216] The rationale for assigning patients to specific lichen planus subtypes and monitoring them in a parallel-group fashion ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com