Method of treating tendinopathy by using interleukin-17 (il-17) antagonist
A tendon, post-treatment technique for the treatment of tendinopathy with interleukin-17 (IL-17) antagonists that addresses weak evidence of long-term efficacy, no efficacy, and controversial benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0212] Example 1 - In tendinopathy, IgG 1 Tissue exposure and effects of anti-IL-17 monoclonal antibodies
[0213] In an in vivo rat disease model, tendinopathy of the rotator cuff tendon was induced by unilateral surgical partial tenotomy of the supraspinatus tendon along the coronal plane. IgG administered subcutaneously or intravenously one week before or one day before surgical induction of tendinopathy 1 Anti-IL-17 monoclonal antibody (CJM112) (15 mg / kg) or vehicle, followed by weekly subcutaneous or intravenous administration for three weeks. Four weeks after the surgical induction of tendinopathy and one week after the last dose of antibody, antibody exposure in rotator cuff tendon tissue, and its pharmacological effects on tendinopathy inflammation and on gait imbalance were assessed. Following tissue homogenization and protein extraction, terminal trough exposure levels of antibodies in rotator cuff tendon tissue, skeletal muscle, and skin were assessed by enzyme-li...
example 2-I
[0217] Ex vivo and in vitro examples of tendon bundle inflammation induced by example 2-IL17
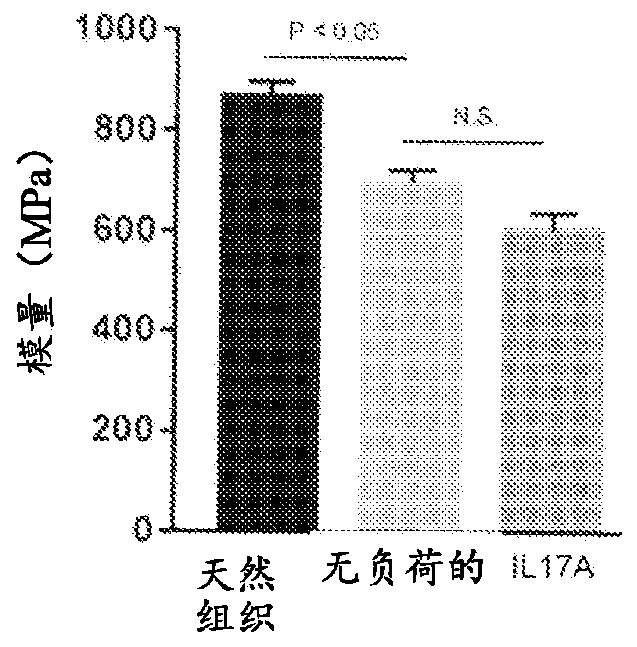
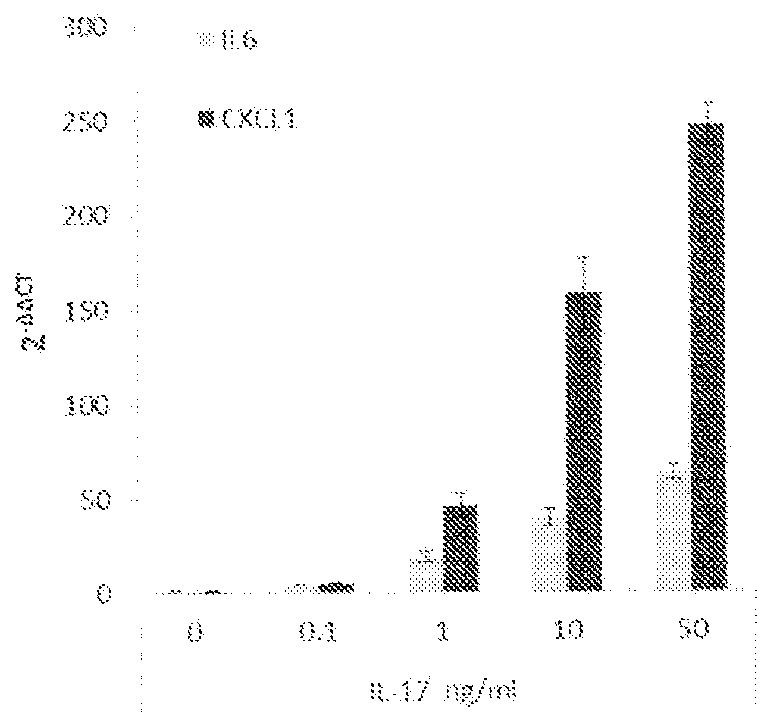
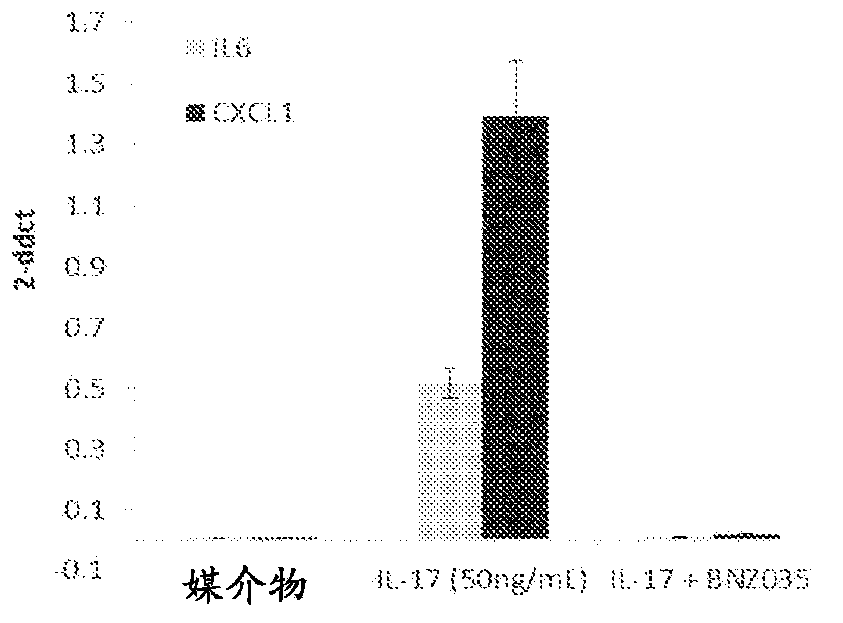
[0218] In an isolated rat tail tendon fascicle model, a model of nonload-induced tendon degeneration, RNASeq analysis revealed hallmarks of intrinsic tendon fascicle inflammation. Unloaded tracts showed >10-fold upregulation of multiple cytokines, chemokines and MMPs including IL6, CXCL1, CCL2, CCL20, CSF1-3, MMP2, 3 and 9. IL-17RA and IL-17RC were found to be highly expressed in tendon bundles, indicating that this tissue is sensitive to IL-17. Computational analysis of signaling pathways from in-house databases identified five pathway hits specific for IL-17 or TH17 cell-specific signatures. Addition of recombinant IL-17A (1.67 nM) to unloaded tendon bundles induced a further increase in the expression of cytokines, chemokines and MMPs (IL-6, CXCL-1, PTGS-2, MMP-3). Simultaneously, and in accordance with tendon degeneration, the tendon marker genes scleraxis and tenomodulin were ...
example 3- 1
[0220]Example 3 - a randomized, double-blind, placebo-controlled, parallel group, phase II, 24-week study investigating patients who were treated with oral NSAID / acetaminophen, physical therapy, or corticosteroid injections Efficacy, safety and tolerability of AIN457 in patients with refractory active overuse tendinopathy
[0221] "Enthesitis" is the term used to describe inflammation of the attachment points of tendons, ligaments, or joint capsules. It is indicated for diseases associated with spondyloarthritis (SpA) including AS and PsA. Enthesitis can be inflammatory or mechanically induced; both can share common features (McGonagle D and Benjamin M (2009) Reports on Rheumatic Diseases Series 6.4:1-6).
[0222] As seen in studies with secukinumab, neutralization of IL-17 has been demonstrated to have efficacy in inflammatory enthesitis of PsA and AS. In study CAIN457F2312, enthesitis was assessed in a subgroup of patients with disease activity at baseline. In this patien...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com