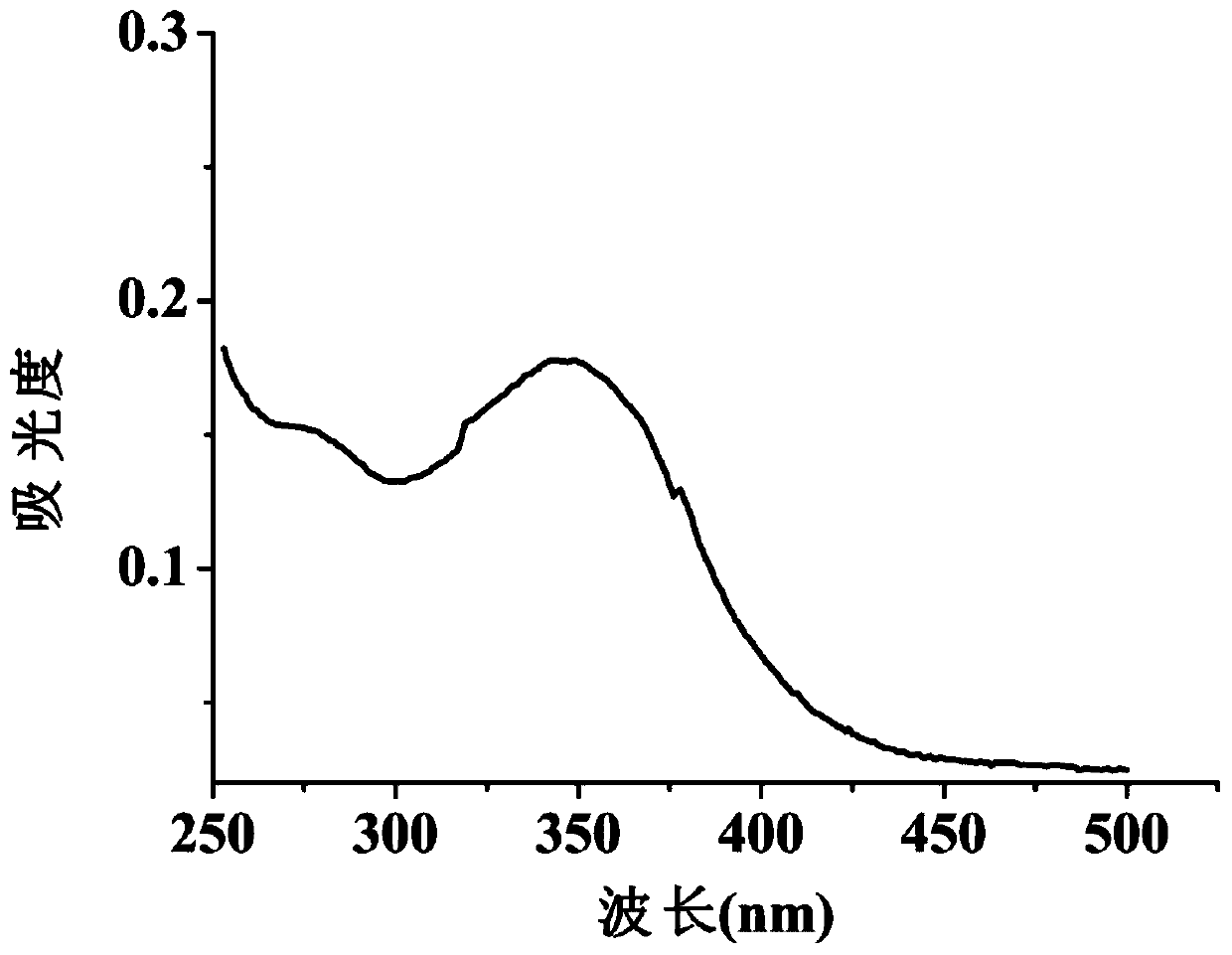
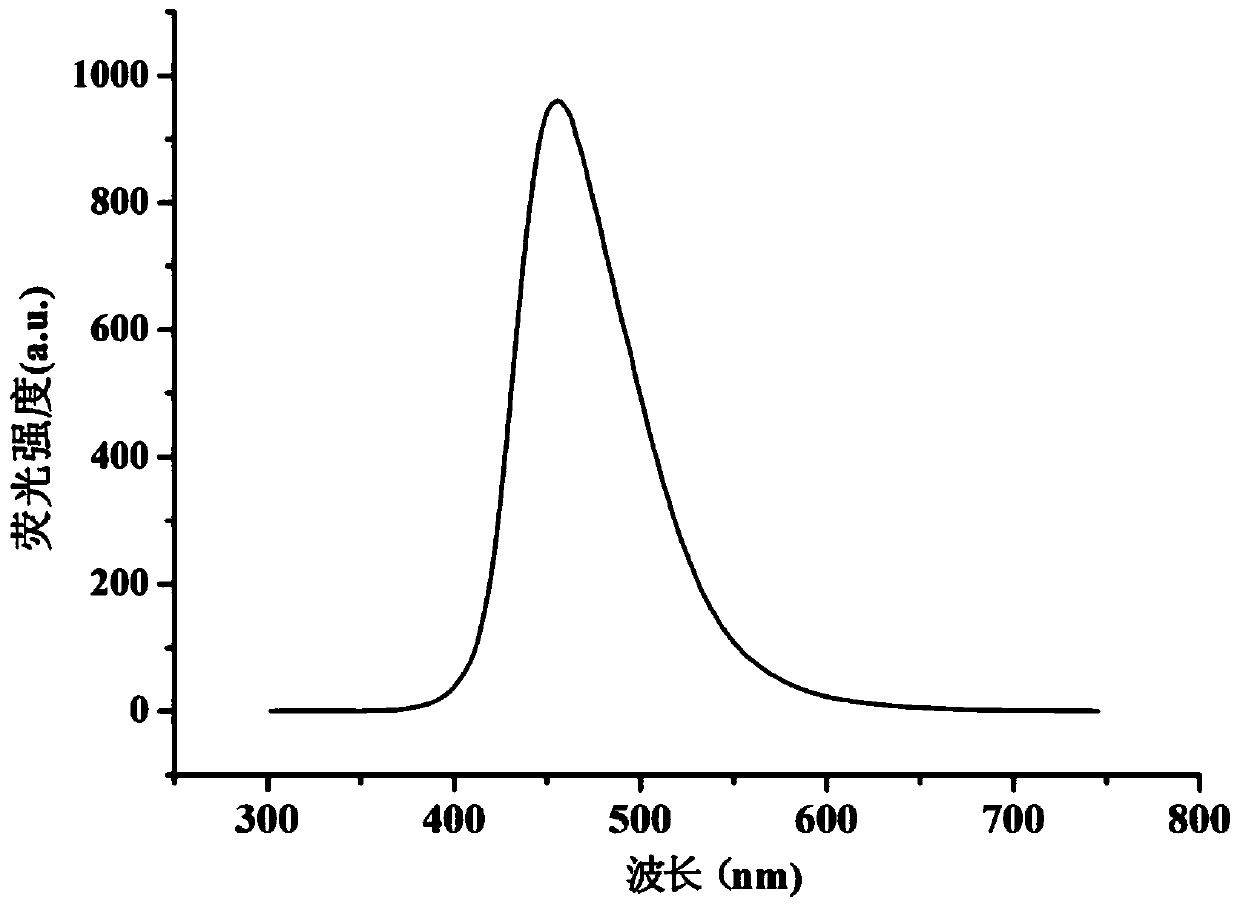
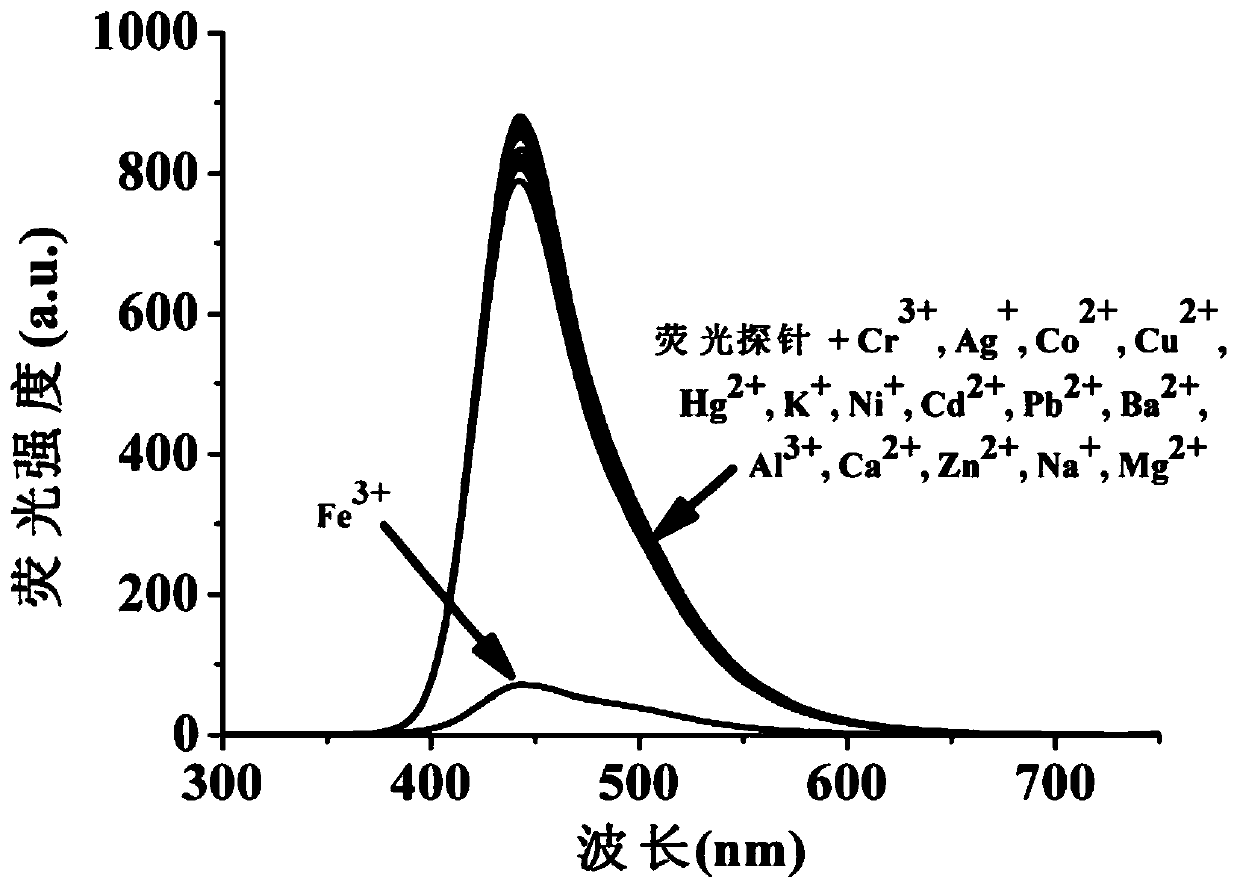
A coumarin fluorescent group ratio fluorescent molecular probe for iron ion detection and its synthesis and use method
A fluorescent molecular probe and fluorescent group technology, which is applied in the field of fluorescent molecular probe synthesis, can solve the problems of unsatisfactory detection limit, metal ion interference, poor sensitivity, etc., and achieves low cost, simple steps, and strong anti-interference ability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0030] Specific embodiment 1: The coumarin fluorescent group ratio fluorescent molecular probe for iron ion detection in this embodiment is 4-methyl-7-hydroxyl-8-[3-(1H-imidazole) propionate- 2-aminomethylene]-2H-benzopyran-2-one, its structural formula is:
[0031]
specific Embodiment approach 2
[0032] Specific embodiment two: the preparation method of the coumarin fluorescent group ratio fluorescent molecular probe for iron ion detection described in specific embodiment one is as follows:
[0033] 1. According to the molar ratio of 4-methyl-7-hydroxyl-8-formyl coumarin and L-histidine is 1: (1~2), the 4-methyl-7-hydroxyl-8-aldehyde Dicoumarin and L-histidine were added to the acidic solvent, refluxed for 4-6 hours, and filtered while hot to obtain the reaction mixture;
[0034] 2. Add the reaction mixture to a mixed solvent of N,N-dimethylformamide and ethanol, heat to dissolve, then cool, and recrystallize to precipitate a solid; filter the solid to obtain 4-methyl-7-hydroxy-8 -[3-(1H-imidazole)propionyl-2-aminomethylene]-2H-benzopyran-2-one, which is the ratio fluorescent molecular detector of coumarin fluorescent group for iron ion detection Needle.
specific Embodiment approach 3
[0035] Embodiment 3: The difference between this embodiment and Embodiment 2 is that the acidic solvent in step 1 is glacial acetic acid, concentrated sulfuric acid or concentrated hydrochloric acid; the others are the same as Embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com