A phenanthroimidazole-coumarin dual fluorescent group ratio fluorescent molecular probe for iron ion detection and its synthesis and use method
A fluorescent molecular probe, phenanthroimidazole technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as inaccurate detection data, achieve simple separation and purification process, strong anti-interference ability, fluorescence The effect of stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
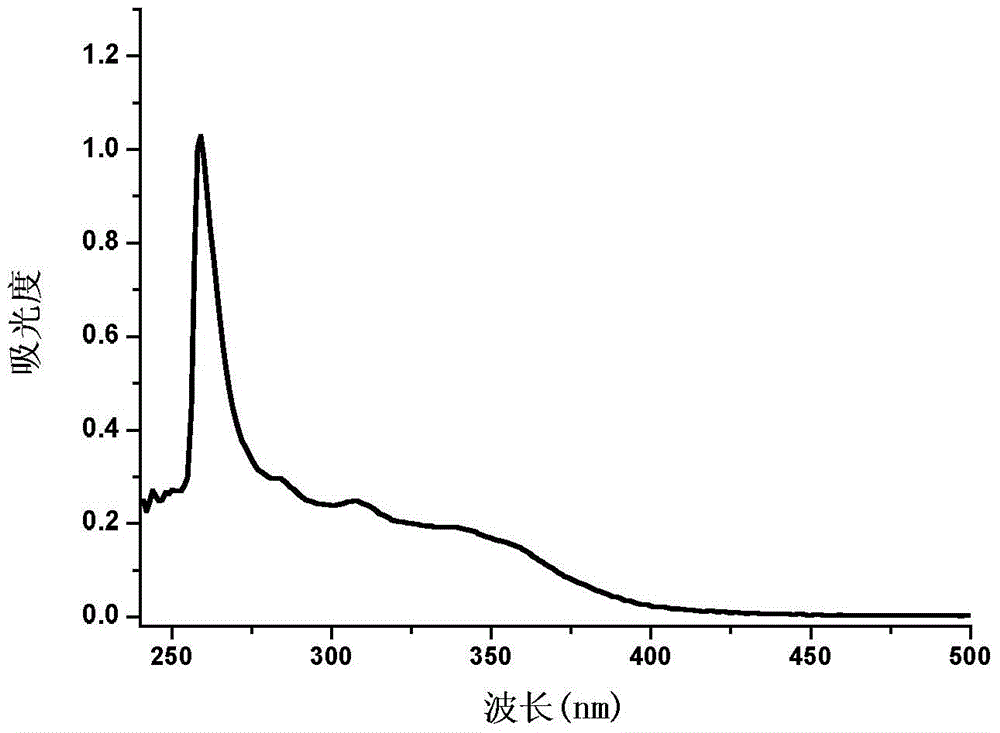
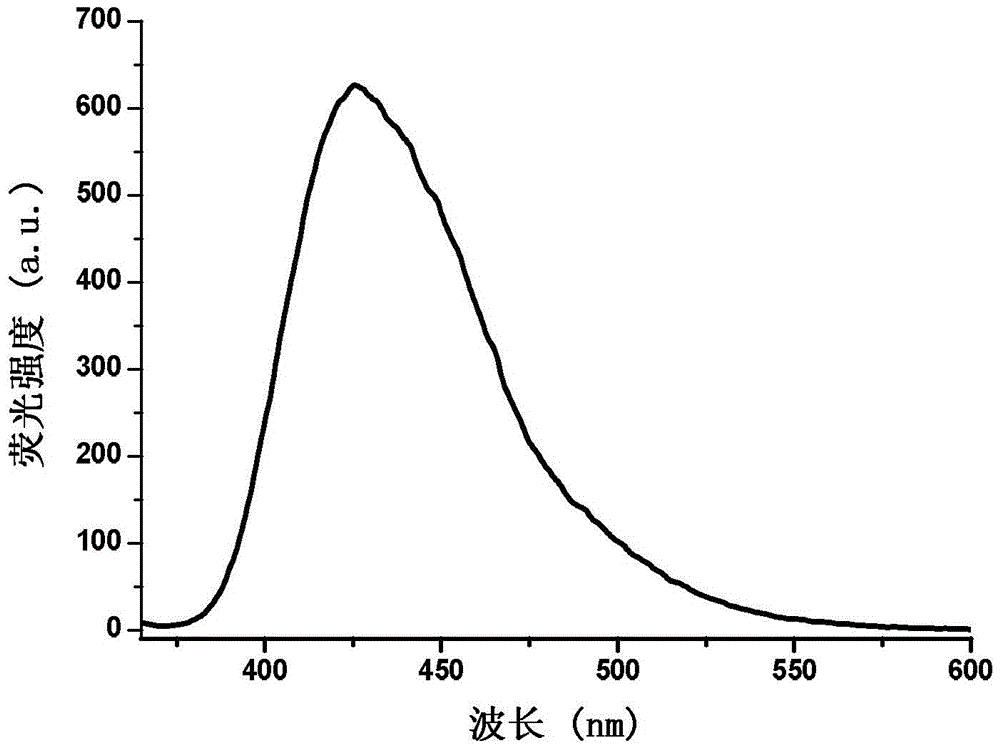
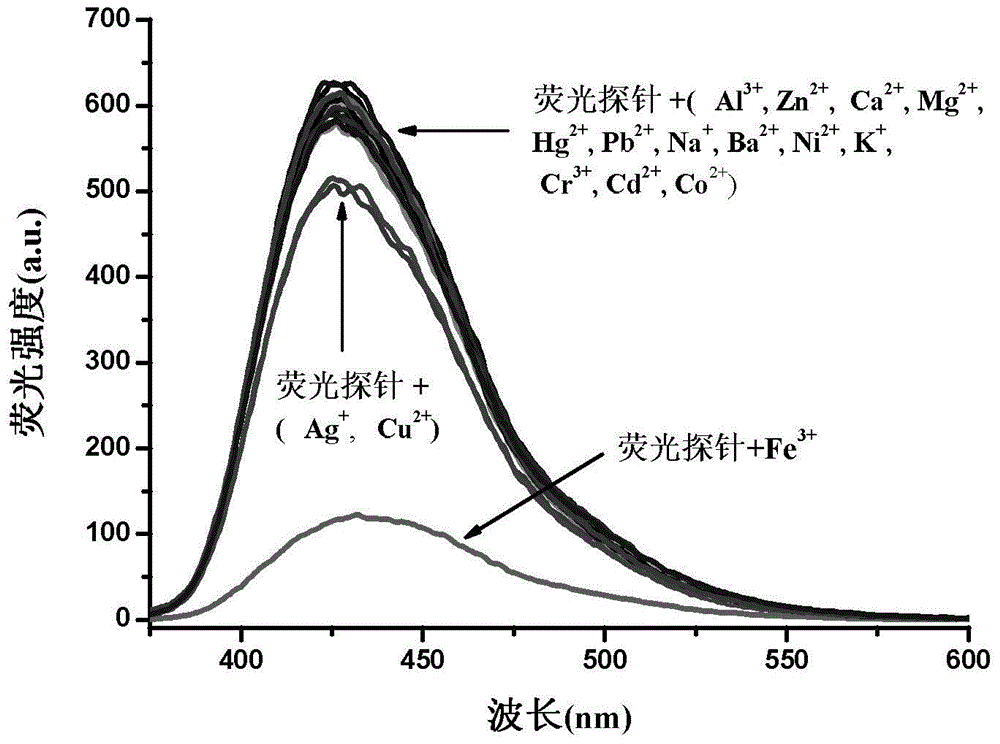
[0031]Specific embodiment one: the phenanthrene imidazole-coumarin dual fluorescent group ratio fluorescent molecular probe for iron ion detection in this embodiment is 4-methyl-7-hydroxyl-8-[2-(1-benzene Base-1H-phenanthrene[9,10-d]imidazole-2-)anilinomethylene]-2H-pyran-2-one, its structural formula is:
[0032]
specific Embodiment approach 2
[0033] Specific embodiment two: the preparation method of the phenanthroimidazole-coumarin dual fluorescent group ratio fluorescent molecular probe for iron ion detection described in specific embodiment one is as follows:
[0034] 1. According to the combination of 1-N-phenyl-2-(2-aminophenyl)-1H-phenanthrene[9,10-d]imidazole and 4-methyl-7-hydroxyl-8-formyl coumarin The molar ratio is 1:(1~5), 1-N-phenyl-2-(2-aminophenyl)-1H-phenanthrene[9,10-d]imidazole and 4-methyl-7-hydroxy -8-formyl coumarin was added into the acidic solvent, stirred and reacted at room temperature for 10-60 minutes to obtain a reaction mixture;
[0035] 2. Pour the reaction mixture into distilled water, adjust the pH value to 8-10, and precipitate a large amount of solid; filter and collect the filter cake;
[0036] 3. Recrystallize the filter cake with ethyl acetate and petroleum ether to obtain 4-methyl-7-hydroxy-8-[2-(1-phenyl-1H-phenanthrene[9,10-d]imidazole-2 -) Anilaminomethylene]-2H-pyran-2-one...
specific Embodiment approach 3
[0037] Specific embodiment three: the difference between this embodiment and specific embodiment two is that the acidic solvent in step one is glacial acetic acid, concentrated sulfuric acid or formic acid; others are the same as specific embodiment two.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com