Liquorice gadoleic acid derivative as well as preparation method and application thereof
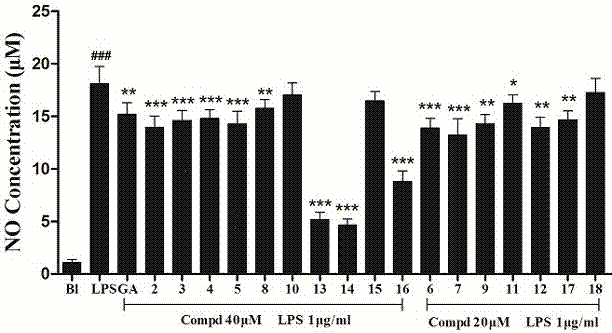
A technology of glycyrrhizic acid and derivatives, which can be used in drug combinations, pharmaceutical formulations, steroids, etc., can solve problems such as tissue damage, and achieve inhibition of the production of inflammatory signaling molecule NO, strong inhibitory activity, and good inflammatory signaling molecule NO. the effect of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~ Embodiment 16
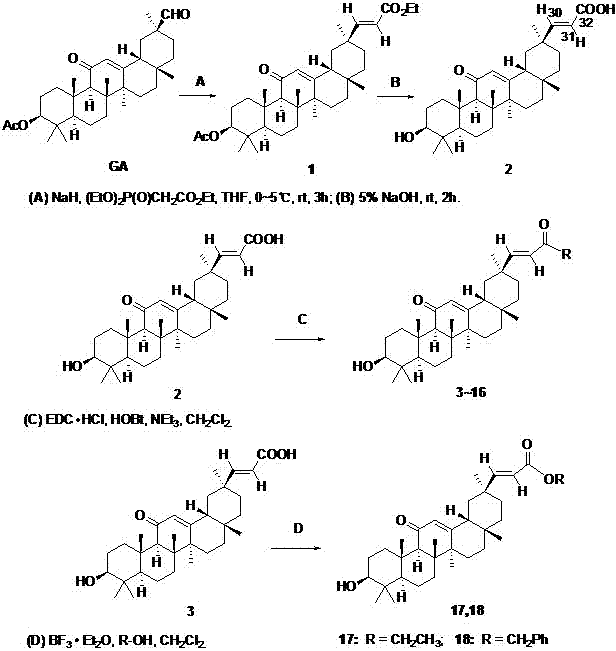
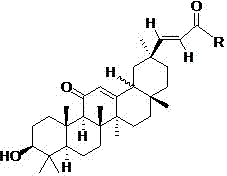
[0044] The preparation of derivative 1~derivative 16 (synthetic route such as figure 1 shown)
[0045] Reference (Beseda I et al., Bioorg. Med. Chem., 2010, 18(1), 433-54), Synthesis of Glycyrrhizin.
[0046]Add 80% sodium hydride (6.0mmol) into anhydrous tetrahydrofuran (100mL), stir at 0°C to 5°C, under nitrogen protection, slowly add triethyl phosphoroacetate (6.0mmol) dropwise over 30 minutes, after the addition is complete , stirred for 1 hour under the same conditions; the obtained glycyrrhizin (5.0mmol) was dissolved in anhydrous tetrahydrofuran (30mL), and was added dropwise to the above mixed reaction solution in 30 minutes under ice-bath conditions, stirred at room temperature for 2 to 3 hours, TLC Monitoring, until the reaction of glycyrrhizin is complete, stop the reaction; filter the reaction solution, wash twice with ethyl acetate, combine the filtrate, extract, wash the organic phase twice with water, dry over anhydrous sodium sulfate, filter and concentrate un...
Embodiment 1
[0051] Derivative 1: white powder, yield 64%, m.p.254-257°C; 1H-NMR (300MHz, CDCl3), δ(ppm): 6.89 (d, 1H, J=16.2Hz, H-30), 5.79 (d,1H,J=16.2Hz,H-31),5.63(s,1H,H-12),4.52(dd,1H,J=11.4,5.0Hz,H-1),4.20(q,2H, J=7.1Hz, OCH2CH3), 2.80(dt, 1H, J=13.5, 3.3Hz, H-5), 2.36 (s, 1H, H-9), 2.05(s, 3H, OAc), 1.38(s, 3H,H-27),1.13(s,3H,H-25),1.12(s,3H,H-26),1.00(s,3H,H-23),1.01(s,3H,H-29) ,0.81(s,3H,H-24),0.80(s,3H,H-28).
Embodiment 2
[0053] Derivative 2: white powder, yield 91%; m.p.259-262°C; 1H-NMR (300MHz, CDCl3), δ (ppm): 7.00 (d, 1H, J=16.2Hz, H-30), 5.82 (d, 1H, J=16.2Hz, H-31), 5.66(s, 1H, H-12), 3.73(q, 2H, J=7.0Hz), 3.24(dd, 1H, J=9.8, 6.5Hz ,H-3),2.79(d,1H,J=13.5,H-18),2.36(s,1H,H-9),1.38(s,3H,H-27),2.34(s,1H,H -9),1.13(s,3H,H-25),1.12(s,3H,H-26),1.00(s,3H,H-23),1.01(s,3H,H-29),0.81( s,3H,H-24),0.80(s,3H,H-28); 13C-NMR(75MHz,CDCl3),δ(ppm):200.3(C-11), 170.43(C-13),169.5( C-32), 157.9 (C-30), 128.3 (C-12), 119.4 (C-31), 78.8 (C-3), 61.8 (C-9), 58.4 (C-22), 54.9 (C -5), 47.4(C-18), 45.4(C-8), 43.3(C-14), 42.8(C-20), 39.1(C-4), 39.1(C-1), 38.0(C- 10), 37.1(C-19), 36.7(C-21), 32.7(C-2), 32.2(C-7), 30.7(C-17), 28.4(C-28), 28.0(C-23 ), 26.4 (C-16), 26.3 (C-15), 23.5 (C-20), 18.6 (C-26), 18.4 (C-27), 17.4 (C-6), 16.3 (C-25) ,15.5(C-24).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com