Pyxinol esterification derivatives having anti-inflammatory activity and preparing method and applications thereof
A technology of derivatives and esterification, which is applied in the field of Pyxinol esterification derivatives and its preparation, can solve serious adverse reactions and other problems, and achieve a strong inhibitory activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (20S,24R)-epoxy-3β-O-(Boc-glycylglycyl)-dammarane-12β,25-diol (1);
[0034] 20S-Protopanaxadiol (8.000g, 17.36mmol) was dissolved in dichloromethane (160mL), m-CPBA (4.490g, 19.51mmol) was added, and stirred at room temperature for 3h. Dilute with chloroform and wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate, and column chromatography to obtain Pyxinol[(20S,24R)-epoxydammarin-3β,12β,25-triol] (5.184g , 10.87mmol, 63%).
[0035] Pyxinol, 1 H NMR (400MHz, CDCl 3 )δ3.84(dd, J=8.8,6.8Hz,1H),3.51(td,J=10.5,4.6Hz,1H),3.18(dt,J=9.9,4.5Hz,1H),2.19(td,J =10.9,3.6Hz,1H),1.28(s,3H),1.27(s,3H),1.14–0.96(m,3H),1.09(s,3H),0.98(s,3H),0.97(s, 3H),0.90(s,3H),0.85(s,3H),0.77(s,3H).
[0036] Pyxinol (42mg, 0.088mmol), N-Boc-2-aminoacetic acid (26mg, 0.148mmol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI, 51mg, 0.266mmol) was dissolved in anhydrous dichloromethane (1.0mL), in an ice bath, added 4-dimethylam...
Embodiment 2
[0039] (20S,24R)–epoxy–3β–O–(Boc–L-alanylglycyl)–dammarane-12β,25–diol (2)
[0040] The intermediate product (iii) (43mg, 0.067mmol) was dissolved in anhydrous TFA (trifluoroacetic acid, 0.5mL), and reacted at room temperature for 10min to complete the reaction. Its concentrate and Boc-L-alanine (28mg, 0.148mmol) were dissolved in anhydrous dimethylformamide (1mL), HBTU (59mg, 0.156mmol) and 4 drops of triethylamine were added, and stirred at room temperature for 4h. Dilute with ethyl acetate, add saturated aqueous sodium bicarbonate to quench the reaction, extract with ethyl acetate, dry the organic phase over anhydrous sodium sulfate, filter, concentrate, and column chromatography to obtain the target product 2 (44mg, 0.062mmol, 93%) , 1 H NMR (400MHz, CDCl 3 )δ4.54(dd, J=8.8,7.2Hz,1H),4.08(dd,J=18.4,4.8Hz,1H),4.02(dd,J=18.4,4.8Hz,1H),4.04-3.95(m ,1H),3.85(dd,J=9.2,5.6Hz,1H),3.51(td,J=10.4,4.4Hz,1H),2.19(td,J=10.1,3.2Hz,1n),1.46(s, 9H),1.28(s,3H),1.27(s,3H),1.10(s,3H),0....
Embodiment 3
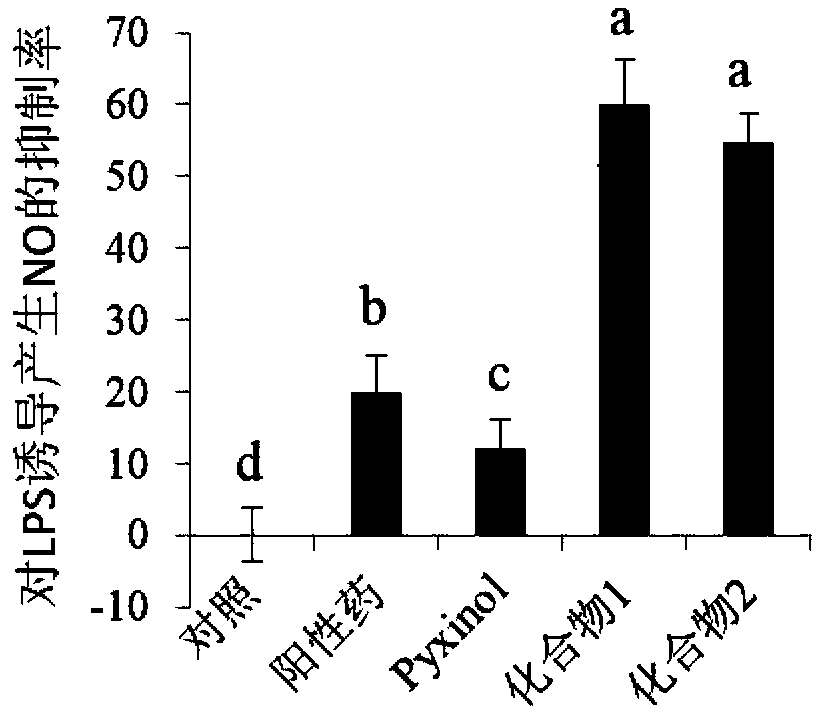
[0042] Detection of inhibitory activity of Pyxinol esterified derivatives on NO production:
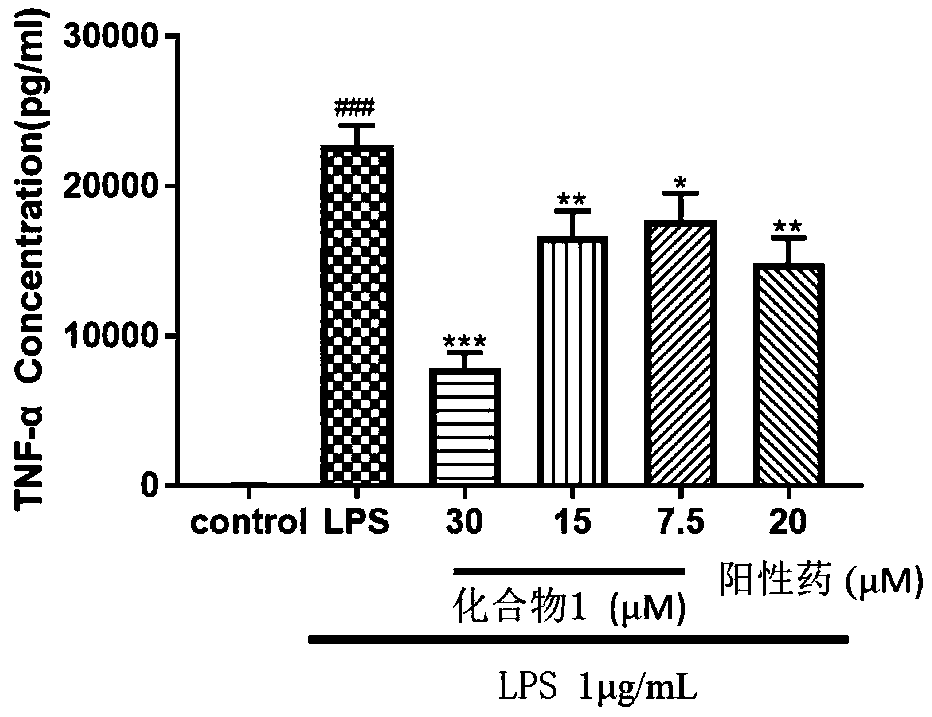
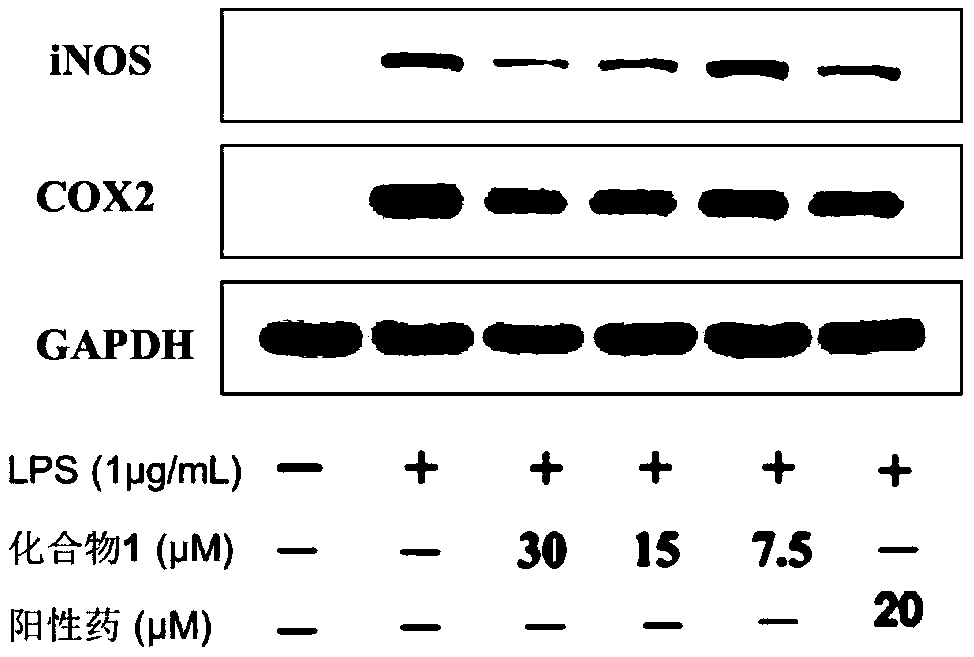
[0043] NO is an important inflammatory mediator. When some pathological changes occur in the body, the amount of NO produced in the body will exceed the normal level. Therefore, NO inhibitors have potential possibilities and opportunities to find new ways to treat inflammation-related diseases. In order to evaluate the anti-inflammatory effect of Pyxinol ester derivatives, the level of NO release induced by lipopolysaccharide (LPS) in RAW264.7 cells was detected by Griess reagent. RAW264.7 cells were treated with 1×10 5 Cells / well were seeded in a 96-well plate and cultured for 6 hours; then the model was stimulated with LPS (1 μg / mL), and the cells were treated with 20 μM derivatives, Pyxinol and positive drug (hydrocortisone sodium succinate) 24 Hours later, use Griess reagent (Beyotime, China) to detect the nitrite level to determine the amount of NO production; then measure the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com