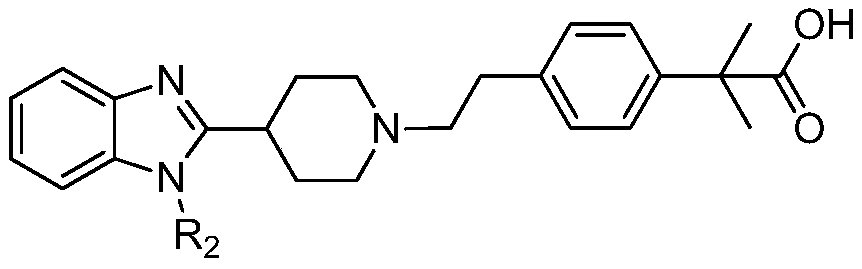
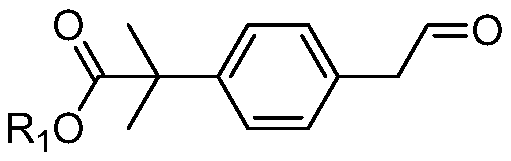
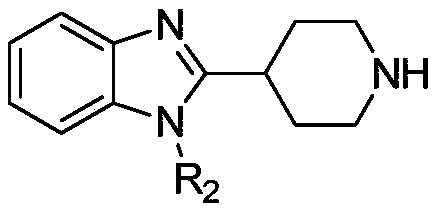
A kind of preparation method and its intermediate of 2-methyl-2'-phenylpropionic acid derivative
A technology of phenylpropionic acid and its derivatives, which is applied in the medical field, can solve problems such as increased raw material costs, purification, and cumbersome process, and achieves the effects of simple and efficient preparation methods, easy industrial production, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: Preparation of 2-methyl-2-(4-(2-hydroxyethyl)-phenyl)-propionic acid methyl ester
[0068] In a reaction vessel, add 2-methyl-2-(4-(2-hydroxyethyl)-phenyl)-propionic acid (5.0 g), methanol (50 mL) and concentrated sulfuric acid (0.5 mL) and heat to 80°C, stirred and reacted for 20 hours, cooled to room temperature, added dropwise saturated sodium carbonate aqueous solution, adjusted the pH to neutral, concentrated until no more liquid dripped, added 30mL water and stirred for 1 hour, added 10mL ethyl acetate for extraction three times, and combined the organic phases , washed with 10 mL of saturated brine, the organic phase was separated, dried with 2 g of anhydrous sodium sulfate for 2 hours, filtered, and the filtrate was concentrated under reduced pressure to obtain 2-methyl-2-(4-(2-hydroxyethyl)-phenyl) - Methyl propionate (5.3 g, 100% yield).
[0069] 1 HNMR, 500MHZ, CDCl 3 , ppm: 1.56(s, 6H), 2.03(t, 1H), 2.82(t, 2H), 3.64(s, 3H), 3.82(t, 2H), 7.1...
Embodiment 2
[0070] Embodiment 2: Preparation of 2-methyl-2-(4-(2-hydroxyethyl)-phenyl)-propionic acid methyl ester
[0071] In a reaction vessel, 2-methyl-2-(4-(2-hydroxyethyl)-phenyl)-propionic acid (2.5 g), dimethylformamide (12.5 mL) and potassium carbonate (5 g ), stirred at room temperature, added dropwise methyl iodide (2.0g), stirred at room temperature for 4 hours, added 30mL of water, stirred for 30 minutes, added 5mL of ethyl acetate for extraction three times, combined the organic phases, washed three times with 10mL of saturated brine, separated organic phase, dried with 1g of anhydrous sodium sulfate for 4 hours, filtered, and the filtrate was concentrated under reduced pressure to obtain 2-methyl-2-(4-(2-hydroxyethyl)-phenyl)-propionic acid methyl ester (2.7g, yield rate 100%).
[0072] 1 HNMR, 500MHZ, CDCl 3 , ppm: 1.56(s, 6H), 2.03(t, 1H), 2.82(t, 2H), 3.64(s, 3H), 3.82(t, 2H), 7.17(d, 2H), 7.27(d, 2H ). MS(ESI):[M+H] + =222.98
Embodiment 3
[0073] Embodiment 3: Preparation of 2-methyl-2-(4-(2-hydroxyethyl)-phenyl)-propionic acid ethyl ester
[0074] In a reaction vessel, add 2-methyl-2-(4-(2-hydroxyethyl)-phenyl)-propionic acid (2.5 g), ethanol (25 mL) and concentrated sulfuric acid (0.3 mL) and heat to 80°C, stirred and reacted for 22 hours, cooled to room temperature, added dropwise saturated sodium carbonate aqueous solution, adjusted the pH to neutral, concentrated until no more dripping, added 10mL water and stirred for 1 hour, added 5mL ethyl acetate for extraction three times, combined the organic phases , washed with 5 mL of saturated brine, separated the organic phase, dried with 1 g of anhydrous sodium sulfate for 3 hours, filtered, and the filtrate was concentrated under reduced pressure to obtain 2-methyl-2-(4-(2-hydroxyethyl)-phenyl) - Ethyl propionate (2.8 g, 100% yield).
[0075] MS(ESI):[M+H] + =237.03
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com