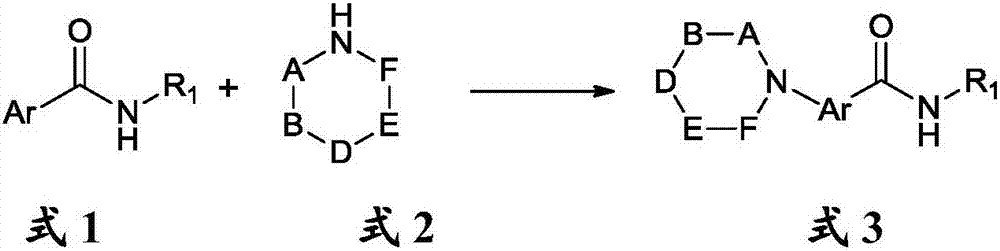
Aromatic-ring C-H bond direct-amination reaction method of aromatic amides
An aryl reaction technology, applied in the field of preparation of aromatic amide compounds, can solve problems such as difficulty, high cost, harsh synthesis conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
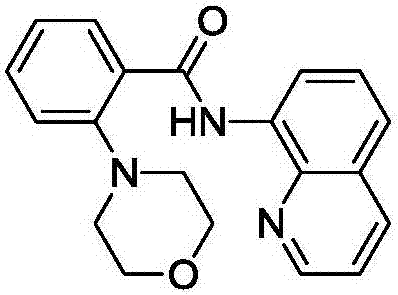
[0051] Embodiment 1: the preparation of 2-morpholine-8-aminoquinoline benzoyl
[0052]
[0053] 0.248 g (1.0 mmol) 8-aminoquinoline benzoyl, 0.26 mL (3.0 mmol) morpholine, 0.1 g (0.5 mmol) Cu(OAc) were added sequentially to the 50 mL reaction tube 2 ·H 2 O, 0.276 grams (2.0 mmol) of potassium carbonate, 5 mL of DMF; the reaction was carried out at 70° C. for 3 hours, after the reaction was completed, it was cooled to room temperature, 30 ml of water was added, and then 0.186 grams (0.5 mmol) of EDTA was added and stirred for 30 minutes. Extract the reaction solution with dichloromethane, combine the organic phases and add a desiccant, filter to remove the desiccant, concentrate the organic phase, and use column chromatography to separate and purify the mixture in the reaction solution to obtain the target product 2-morpholine-8 -Aminoquinoline benzoyl 0.300 g (90% yield).
Embodiment 2
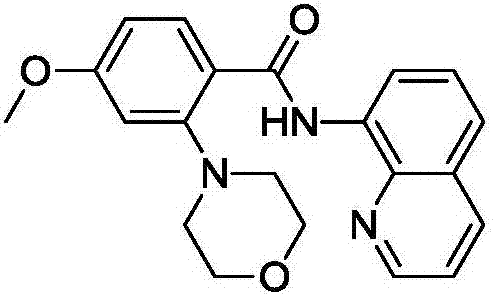
[0054] Embodiment 2: Preparation of 2-morpholine-4-methoxy-8-aminoquinoline benzoyl
[0055]
[0056] According to the method and steps described in Example 1, the difference is that 8-aminoquinoline benzoyl is changed to 4-methoxy-8-aminoquinoline benzoyl. Finally, 0.290 g of the target product was obtained (yield 80%).
Embodiment 3
[0057] Embodiment 3: Preparation of 2-morpholine-4-methyl-8-aminoquinoline benzoyl
[0058]
[0059] According to the method and steps described in Example 1, the difference is that 8-aminoquinoline benzoyl is changed to 4-methyl-8-aminoquinoline benzoyl. Finally, 0.288 g of the target product was obtained (yield 83%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com