Nilotinib as a drug for the treatment of dengue virus infection and its pharmaceutical use
A technology of dengue virus and nilotinib, which is applied in the directions of antiviral agents, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problem of no dengue virus and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1 Nilotinib toxicity test to BHK-21 cells
[0020] BHK-21 cells (baby hamster kidney cells) are DENV2 susceptible cells. The BHK-21 cells used in the experiment belonged to our department; MTT was purchased from Beyontian Institute of Biotechnology; fetal bovine serum was purchased from American GIBICO Company; cell culture plates were purchased from American Corning Company; RPMI 1640 medium was purchased from American GIBICO Company.
[0021] The experimental steps are as follows:
[0022] 1) Inoculation of BHK-21 cells: use RPMI 1640 medium containing 10% (V / V) fetal bovine serum to prepare a single cell suspension, and inoculate 10,000 cells per well into a 96-well cell culture plate;
[0023] 2) Culture BHK-21 cells: at 37°C, 5% (V / V) CO 2 Cultivate under culture conditions for 24 hours;
[0024] 3) Add Nilotinib: Aspirate and discard the medium in each well, add 100 μl of Nilotinib diluted to the corresponding concentration with the RPMI 1640 medium of ...
Embodiment 2
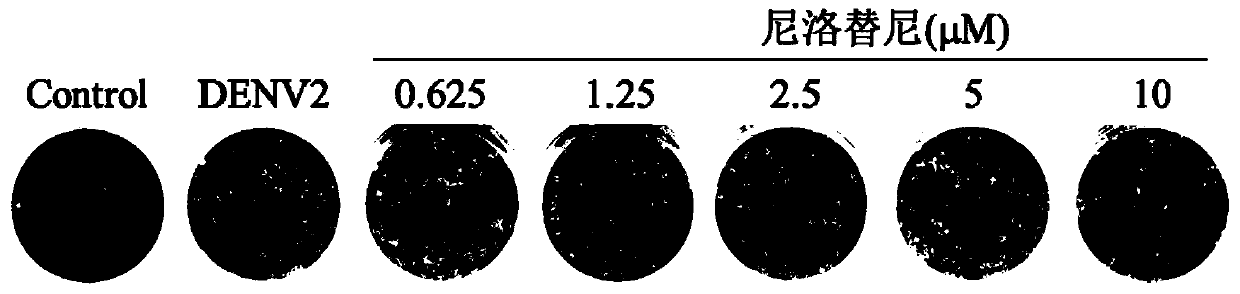
[0032] Example 2 Nilotinib inhibits DENV2 infection of BHK-21 cells:
[0033] BHK-21 is used as the cell for culturing virus DENV2, 10TCID50, the experimental steps are as follows:
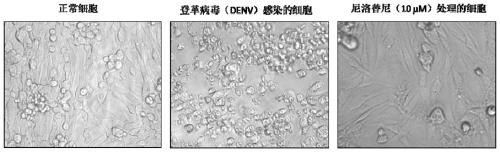
[0034] 1) Add BHK-21 to the cell culture plate. After 24 hours, the cells grow to a monolayer, and the cells cover about 80% to 90% of the bottom of the well. Aspirate the medium, wash once with PBS, and insert 200 μl of virus sample , adsorption at 37°C for 1 hour. After the adsorption was completed, the virus liquid in each well was discarded and washed once with PBS. Add the specified concentration of nilotinib diluted in RPMI 1640 medium containing 10% (V / V) fetal bovine serum, and store at 37°C in 5% (V / V) CO 2 cultured under culture conditions. After 96 hours, after the cells showed obvious cytopathic changes, the cytopathic effects were observed under a microscope. See figure 1 .
[0035] 2) Collect the supernatant and measure the content of lactate dehydrogenase (LDH) released by the...
Embodiment 3
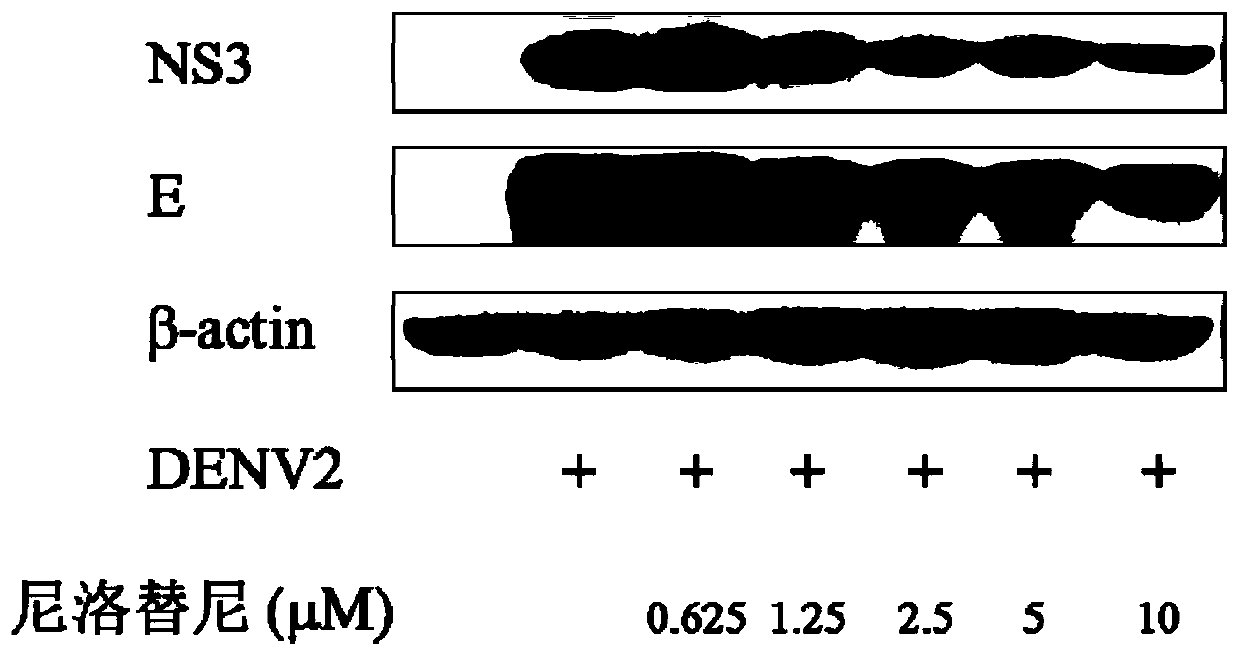
[0042] Example 3 Inhibition test of nilotinib on DENV2 key protein NS3 and E protein
[0043] 1) Collection of total cell protein: After BHK-21 cells were treated with drugs and dengue virus (DENV2), the protein was extracted for 48 hours and collected in a 1.5ml Eppendorf tube.
[0044] 2) Detection of sample protein concentration: the protein standard was diluted with double distilled water to a gradient concentration of 0, 0.0008, 0.0016, 0.0032, 0.004, 0.006, 0.008mg / ml; the protein concentration in the sample was calculated according to the standard curve. Make the protein concentration consistent for each sample.
[0045]3) Western blot detection of dengue virus protein expression differences after gradient concentration nilotinib treatment: add various reagents in order, and start electrophoresis with 80V constant voltage for electrophoresis. After the front of the dye enters the separating gel, change 120V, according to the degree of separation of the pre-stained prot...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com