Enhancer RNA combination used for diagnosing unexplained recurrent spontaneous abortion, primer group and application and kit thereof
A technology for recurrent miscarriage and unclear diagnosis, applied in the field of genetic engineering, to achieve the effects of wide detection spectrum, high sensitivity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
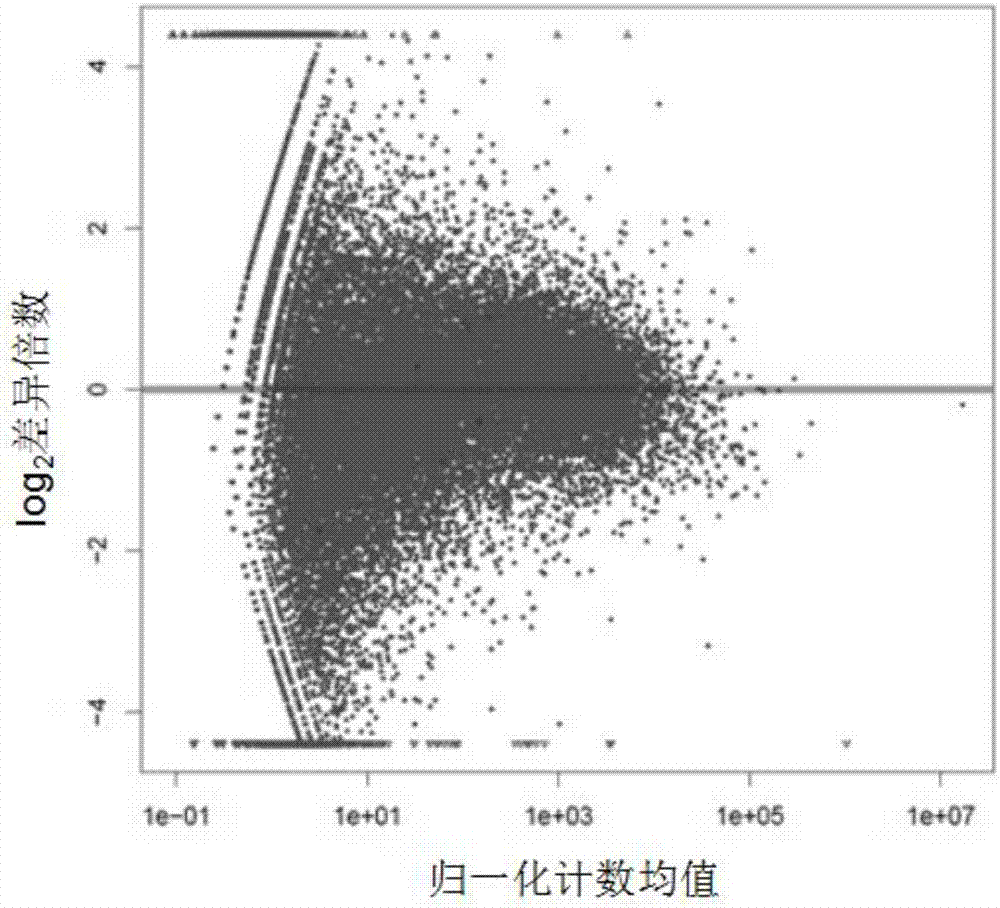
[0040] Example 1: Screening, Screening and Confirmation of Enhancer RNA in Unexplained Recurrent Miscarriage
[0041] 1. Materials
[0042] 1.1 Organization
[0043] The clinical tissue samples of the present invention are all from patients with unexplained recurrent abortion diagnosed in Huai'an First People's Hospital Affiliated to Nanjing Medical University from March 2015 to July 2016. Exact match. The use of all specimens was signed by the patients or their trustees, and the standards of the Medical Ethics Committee of Nanjing Medical University were obtained.
[0044] 1.2 Reagents
[0045] TRIzol TM Reagent was purchased from Invitrogen, USA. The reverse transcription kit (DRR036A) was purchased from Takara, Japan. The SYBR reagent used in real-time quantitative PCT was purchased from Nanjing Nuoweizan Biotechnology Co., Ltd. PCR primers were designed and synthesized by Shanghai Yingjun Biotechnology Co., Ltd. Transcriptome sequencing and data analysis were comp...
Embodiment 2
[0052] Example 2: qRT-PCR method to verify transcriptome sequencing results
[0053] 1. Materials
[0054] 1.1 Organization
[0055] The clinical tissue samples of the present invention are the same as in Example 1.
[0056] 1.2 Reagents
[0057] The reagents used in qPCR are the same as in Example 1.
[0058] 2. Method
[0059] 2.1 Preparation of cDNA samples
[0060] Grind the tissue in liquid nitrogen, add 1 mL TRIzol for every 50-100 mg tissue, and use a homogenizer for homogenization. The sample volume should not exceed 10% of the volume of TRIzol. Place the homogenized sample at room temperature (15-30°C) for 5 minutes to completely separate the nucleic acid-protein complex. Add 0.2 mL of chloroform for every 1 mL of TRIzol used, shake vigorously for 15 s, and place at room temperature for 3 min. Centrifuge at 10 000 g for 15 min at 4°C. The sample is divided into 3 layers: the bottom layer is a red organic phase, the upper layer is a colorless aqueous phase and...
Embodiment 3
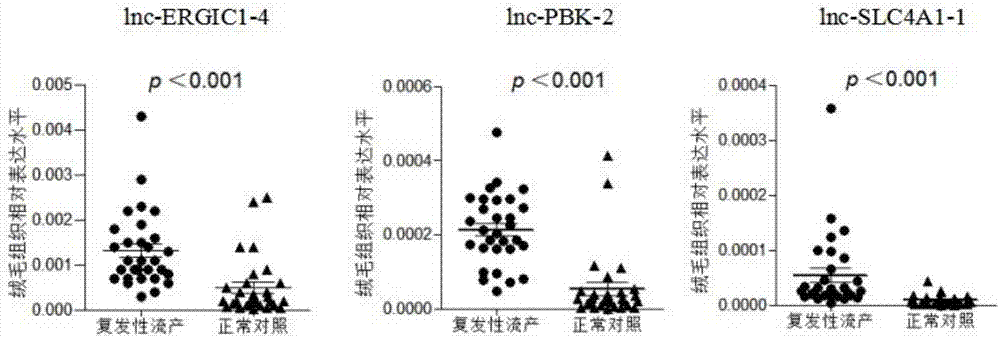
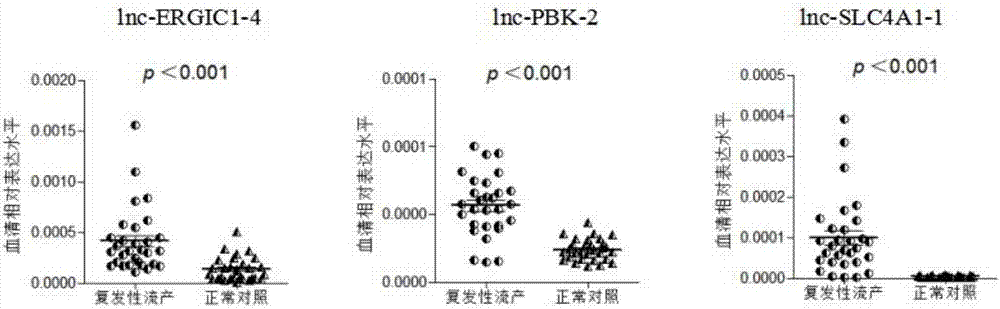
[0071] Example 3: qRT-PCR method to detect the expression of enhancer RNA lnc-ERGIC1-4, lnc-PBK-2, lnc-SLC4A1-1 in serum
[0072] 1. Materials
[0073] 1.1 Serum
[0074] The collection of clinical serum samples of the present invention is the same as in Example 1.
[0075] 1.2 Reagents
[0076] The reagents used in qPCR are the same as in Example 1.
[0077] 2. Method
[0078] Add 1 mL TRIzol to 200 μl serum sample, mix thoroughly, and let stand at 4°C for 5 minutes to completely separate the nucleic acid-protein complex. Subsequent methods are the same as in Example 1 2.2.
[0079] 3. Results
[0080]Compared with normal pregnant women, lnc-ERGIC1-4, lnc-PBK-2, and lnc-SLC4A1-1 were all highly expressed in the serum of recurrent patients (such as image 3 shown), suggesting that this group of enhancer RNAs is associated with unexplained recurrent miscarriage and can be used for the diagnosis of the disease.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap