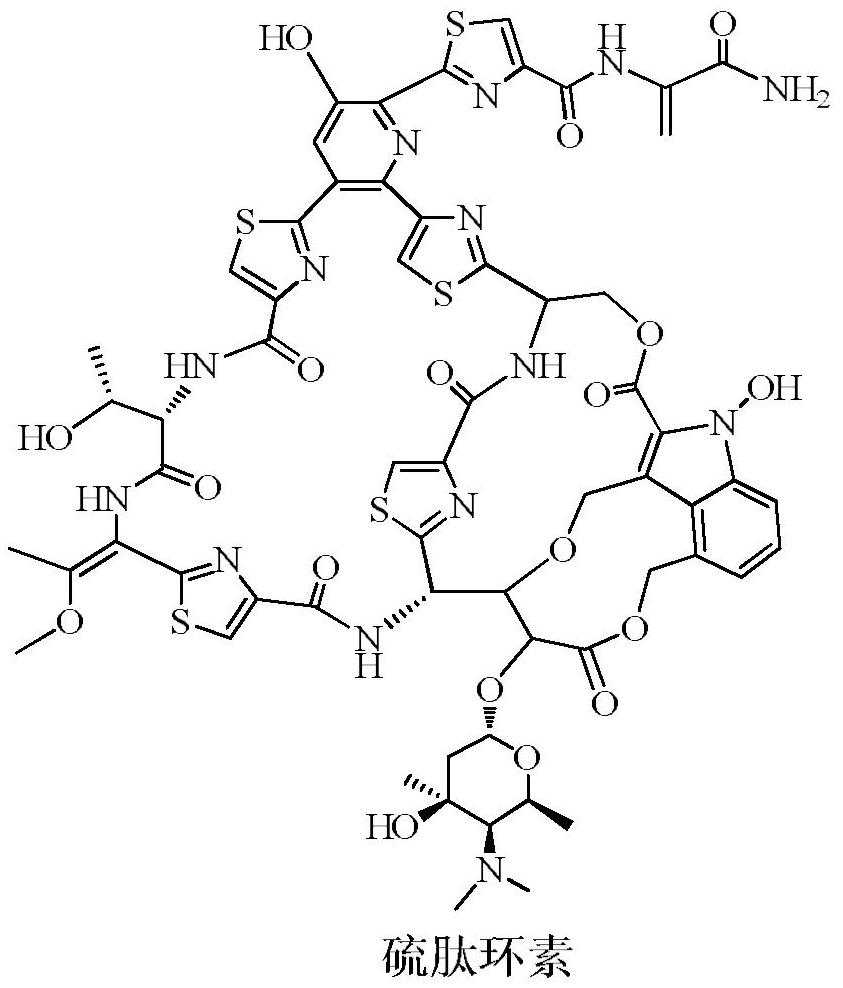
A kind of pharmaceutical composition of Thiopeptidecycline
A technology of thiopeptidecycline and its composition, which is applied in the field of thiopeptidecycline pharmaceutical composition, can solve the problems of drug efficacy or safety that cannot be successfully developed into medicines, and achieve strong anti-(drug-resistant) bacteria activity, A wide variety of effects with significant application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 Thiopeptidecycline HPLC detection method and standard curve
[0037] Thiopeptidecycline bulk drug needs to be dissolved in DMSO to 1 mg / mL, and the pharmaceutical composition needs to be diluted to 1 mg / mL with the corresponding solution for detection.
[0038] Chromatographic column: Waters Atlantis C18 chromatographic column (4.6×150mm, 5μm)
[0039] Mobile phase A: pure medium containing 1‰TFA
[0040] Mobile phase B: 1‰TFA in acetonitrile
[0041] Mobile phase elution gradient:
[0042] time (min) 0 30 31 34 Mobile phase B% 30 60 90 90
[0043] Flow rate: 1.0mL / min
[0044] Detection wavelength: 362nm
[0045] Injection volume: 10μL
[0046] Column temperature: 40°C
[0047] Sample concentration: generally determined as 1mg / mL
[0048] Under the above-mentioned chromatographic conditions, the peak shape of thiopepticycline is good, and the retention time of thiopepticycline is about 17.5min.
[0049] Precisely weigh th...
Embodiment 2
[0051] The examples of thiopeptidecycline compositions listed in this patent are only for illustration and do not limit the scope of application of the present invention.
[0052] Weigh 500 mg of thiopeptidecycline powder, put it in a 100ml beaker, add 7ml of water for injection, stir well, adjust the pH value to 2.0-4.0 with different pH regulators, stir to dissolve thiopeptidecycline, add water for injection to 10ml, and centrifuge Remove the insoluble matter, and after diluting with water for injection, HPLC detects the degradation of the main drug and determines the dissolution. It was tested that the main drug thiopeptidecycline did not degrade, and the solubility of thiopeptidecycline at pH 2.0-4.0 was basically the same under the action of a specific pH regulator. When the pH regulator is hydrochloric acid, the solubility of thiopeptidecycline is 0.35mg / mL; when the pH regulator is citric acid, the solubility is 0.27mg / mL; when the pH regulator is acetic acid, the solub...
Embodiment 3
[0054] The examples of thiopeptidecycline compositions listed in this patent are only for illustration and do not limit the scope of application of the present invention.
[0055] Weigh 500 mg of thiopeptidecycline powder, put it in a 100ml beaker, add 7ml of water for injection, stir well, adjust the pH value to 5.0 with different pH regulators, stir to dissolve thiopeptidecycline, add water for injection to 10ml, and centrifuge to remove insoluble After being diluted with water for injection, HPLC detects the degradation of the main drug and measures the dissolution. It has been tested that the main drug thiopeptidecycline has not been degraded. When the pH regulator is hydrochloric acid, the solubility of thiopeptidecycline is 0.11mg / mL; when the pH regulator is citric acid, the solubility is 0.06mg / mL; when the pH regulator is acetic acid, the solubility is 0.07mg / mL; when the pH When the regulator is malic acid, the solubility is 0.11mg / mL; when the pH regulator is nitri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com