Chimeric antigen receptor and expression gene thereof, double-antigen regulated type T cell modified by chimeric antigen receptor, and application thereof
A technology of chimeric antigen receptors and gene expression, applied to genetically modified cells, cells modified by introducing foreign genetic material, receptors/cell surface antigens/cell surface determinants, etc., can solve poor specificity and side effects of treatment Waiting for questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
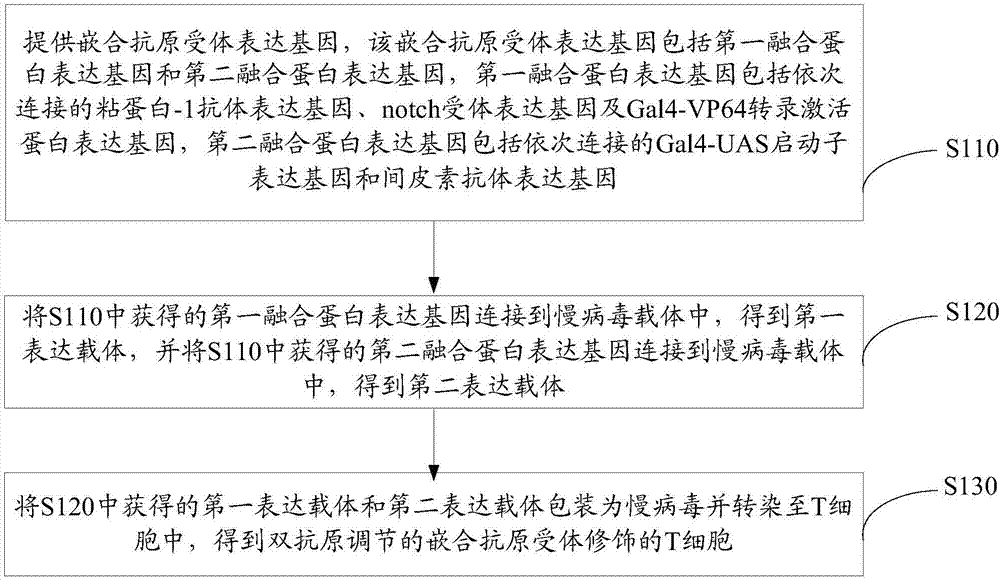
[0086] see image 3 , a method for preparing chimeric antigen receptor-modified T cells regulated by dual antigens according to one embodiment, comprising the following steps S110-S130.
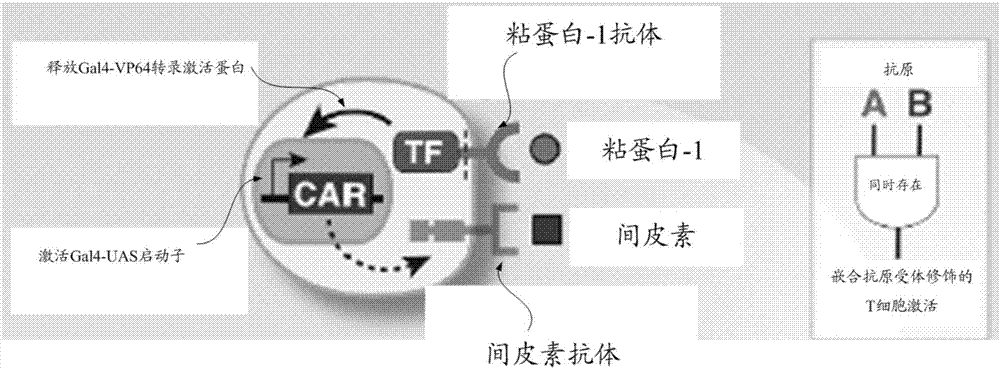
[0087] S110. Provide a chimeric antigen receptor expression gene, the chimeric antigen receptor expression gene includes a first fusion protein expression gene and a second fusion protein expression gene, the first fusion protein expression gene includes mucin-1 antibody expression linked in sequence gene, notch receptor expression gene and Gal4-VP64 transcription activator protein expression gene, and the second fusion protein expression gene includes Gal4-UAS promoter expression gene and mesothelin antibody expression gene connected in sequence.
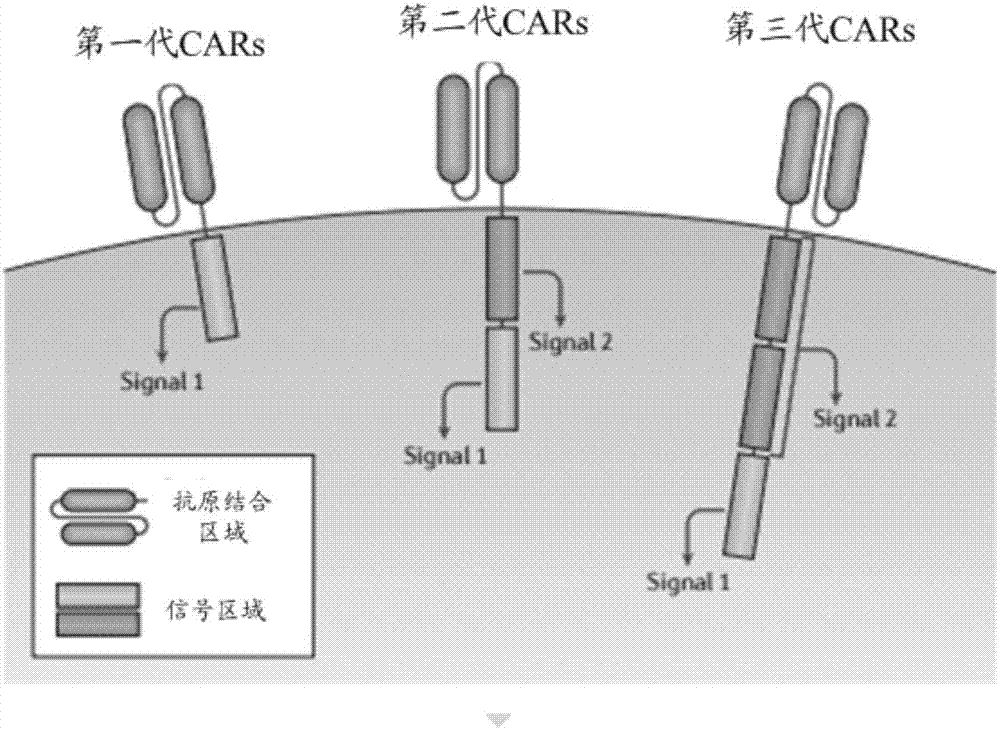
[0088] Specifically, the structure of the chimeric antigen receptor (CAR) can be referred to above, and will not be repeated here.
[0089] In one embodiment, the second fusion protein further includes a terminator linked to the mesothelin antibo...
Embodiment 1
[0103] Preparation of chimeric antigen receptor-modified T cells regulated by dual antigens
[0104] Synthesize the first fusion protein expression gene (including the nucleotide sequence shown in SEQ ID No.1, the nucleotide sequence shown in SEQ ID No.2 and SEQ ID No. The nucleotide sequence shown in No. 3. The expression of the first fusion protein is anti muc-1-synNotch-Gal4VP64.
[0105] The expression gene of the first fusion protein was double digested by EcoR I and BamH I, and cloned into the lentiviral vector pLV-IRES-Puro to obtain the first expression vector. The constructed first expression vector EcoR I and BamH I were double digested and identified, and the electrophoresis of the digested product was as follows: Figure 4 shown (where Figure 4 The 2.4kb fragment is the target fragment, and the 8.1kb fragment is the vector itself). The size of the target fragment was in line with expectations, indicating that the vector was successfully constructed.
[0106] S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com