Triazolyl pyrimidinone compounds as PDE2 inhibitors
一种化合物、溶剂化物的技术,应用在抑制剂的化合物领域,能够解决缺乏功效、药物不依从、不耐受副作用等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0027]
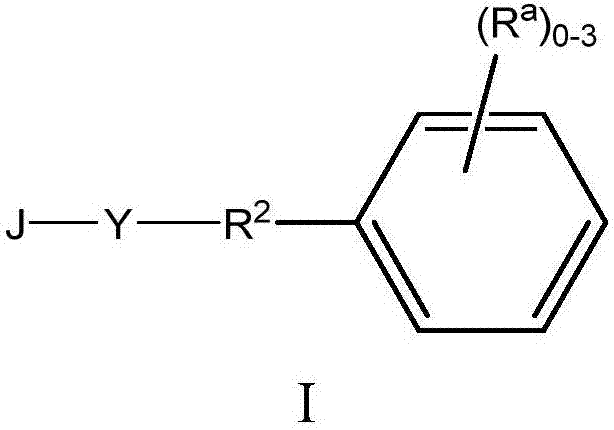
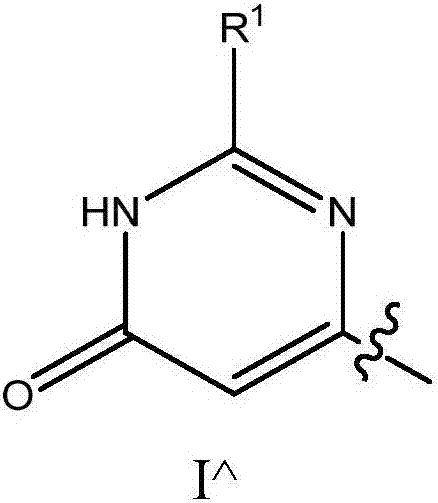
[0028] where R 1 Choose from H, C 1-6 Alkyl, C 2-6 Alkenyl, (CH 2 ) n C 3-10 Cycloalkyl, and (CH 2 ) n C 6-10 Aryl, the alkyl and aryl are optionally replaced by 1 to 3 R a group substitution.
[0029] When Y is triazolyl, one of its nitrogen atoms is attached to R 2 And when one of its carbon atoms is attached to J, another embodiment of formula I of the present invention is realized. When Y is a triazolyl group wherein one of its nitrogen atoms is attached to J and one of its carbon atoms is attached to R 2 When, realize another embodiment of the formula I of the present invention.
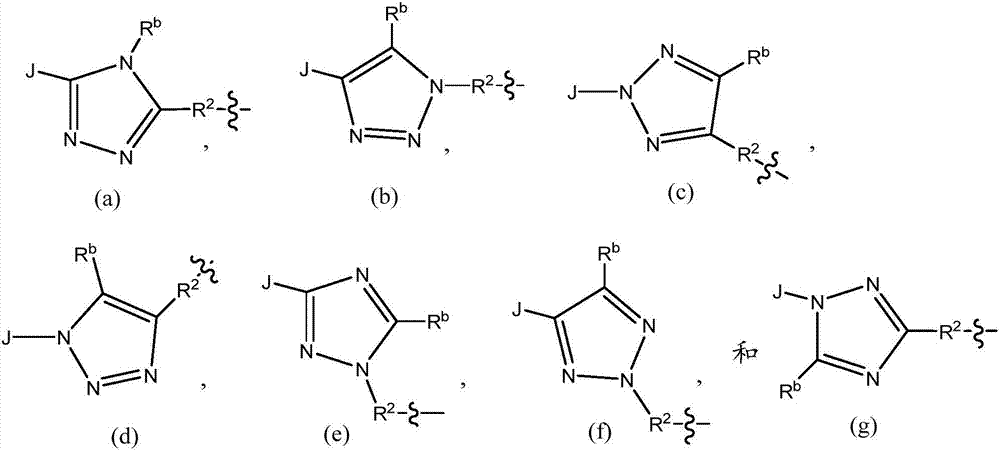
[0030] When Y is a triazolyl group selected from the following groups,
[0031]
[0032] where R 2 and R b Another embodiment of formula I of the present invention is realized when as originally described, and the ~ line represents the point of attachment.
[0033]When Y is (a), (b), (c), (d), (e), (f) or (g) and R b When is hydrogen, one aspect of this sub-embodim...
preparation Embodiment 1 and 2
[0261]
[0262] 2-(Cyclopropylmethyl)-4-ethynyl-6-methoxy-pyrimidine and (E)-2-(but-1-enyl)-4-ethynyl-6- Methoxypyrimidine (Scheme 1).
[0263] Step 1. 2-(cyclopropylmethyl)pyrimidine-4,6-diol: To a mixture of NaOMe (4.95 g, 92.0 mmol) in methanol (80 mL) was added 2-cyclopropylacetamidine hydrochloride (6.00 g, 44.8 mmol) at room temperature. The reaction mixture was stirred at room temperature for 5 minutes, then dimethyl malonate (5.91 g, 44.8 mmol) was added. The reaction mixture was stirred at 65°C for 16 hours. The resulting mixture was cooled and filtered. The filter cake was washed with methanol (80 mL). The combined filtrates were diluted with water (320 mL). The pH of the mixture was adjusted to 2 with 5M aqueous HCl. The mixture was then filtered. The filter cake was washed with diethyl ether (20 mL) and dried to give the title compound as a solid, which was used in the next step without further purification. MS = 167.1 (M+1).
[0264] Step 2. 4,6-d...
preparation Embodiment 6
[0271]
[0272] 4-Methoxy-2-methyl-6-(2H-1,2,3-triazol-4-yl)pyrimidine (Scheme 1).
[0273] 4-methoxy-2-methyl-6-(2H-1,2,3-triazol-4-yl)pyrimidine: To azido-trimethylsilane (1.17g, 10.1mmol) and 4-ethynyl-6-methoxy-2-methylpyrimidine (1.00g, 6.75mmol) in DMF (13.5mL) and MeOH (1.5 mL) was added CuI (0.129 g, 0.7 mmol). The reaction mixture was purged 3 times with nitrogen, sealed and stirred at 100°C for 4 hours. The resulting mixture was cooled to room temperature and concentrated in vacuo. The residue was purified by silica gel chromatography (50% ethyl acetate in petroleum ether) to afford the title compound. MS = 192.0 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com