Uses of thiazole-based compound as antibacterial synergist
A technology of compounds and uses, applied in the field of medicine, can solve problems such as macro-nephrotoxicity and neurotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0139] Preparation method of the compound of the present invention
[0140] The compound represented by the general formula (I) of the present invention can be prepared by the following method, but the conditions of the method, such as reactant, solvent, base, amount of compound used, reaction temperature, reaction time required, etc. are not limited to the following Explanation. The compounds of the present invention can also be conveniently prepared by combining various synthetic methods described in this specification or known in the art, and such combinations can be easily performed by those skilled in the art to which the present invention belongs.
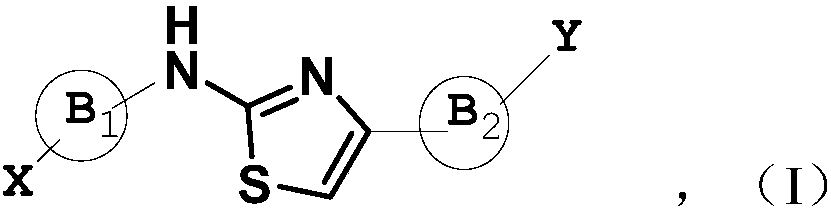
[0141] The compound represented by the general formula (I) of the present invention can be synthesized by the following method:
[0142] (1) In an inert solvent, a compound of formula A and a compound of formula B are reacted at a certain temperature (such as reflux) to obtain a compound of formula C. The compound of formula C rea...
Embodiment 1-30
[0165] Example 1-30: Preparation of Compound
Embodiment 1
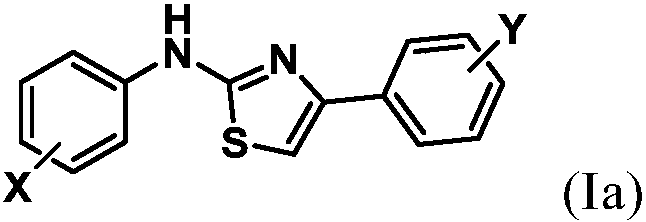
[0166] Example 1 2-(4-Trifluoromethylbenzimidyl)-4-(4-methylphenyl)thiazole
[0167] 1.1 Preparation of N-(4-trifluoromethylphenyl)thiourea
[0168]
[0169] In a 100mL round-bottom flask, 12mmol of benzoyl chloride and 20mL of acetone were added, and then 12mmol of potassium thiocyanate was added. A large amount of white solid was formed in the reaction. After 15 minutes, when the reaction is over, the solid is filtered, and the filtrate is spin-dried to obtain a pale yellow liquid, which is directly put into the next reaction. A 100mL round bottom flask was charged with 10mmol of 4-trifluoromethylaniline, 30mL of ethyl acetate was dissolved, and the product prepared in the previous step was added to this solution, and heated to reflux for reaction. After the reaction was followed by TLC, the reaction solution was cooled to room temperature, the solvent was evaporated under reduced pressure, 10 mL ethanol and 10 mL 2N sodium hydroxide solution were directly added, and the reactio...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap