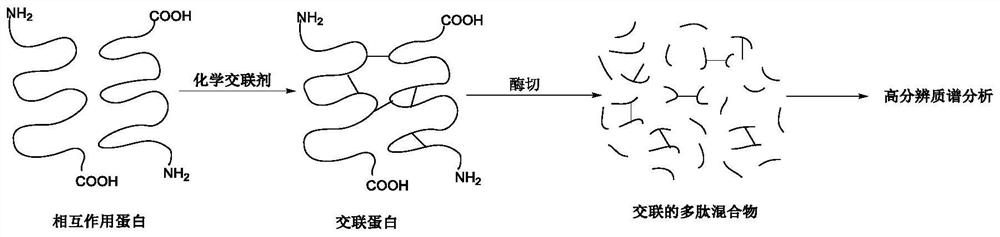
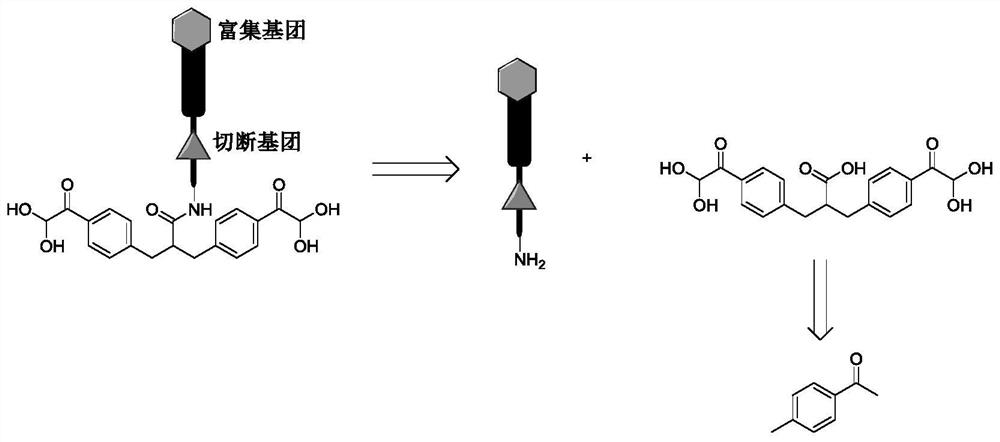
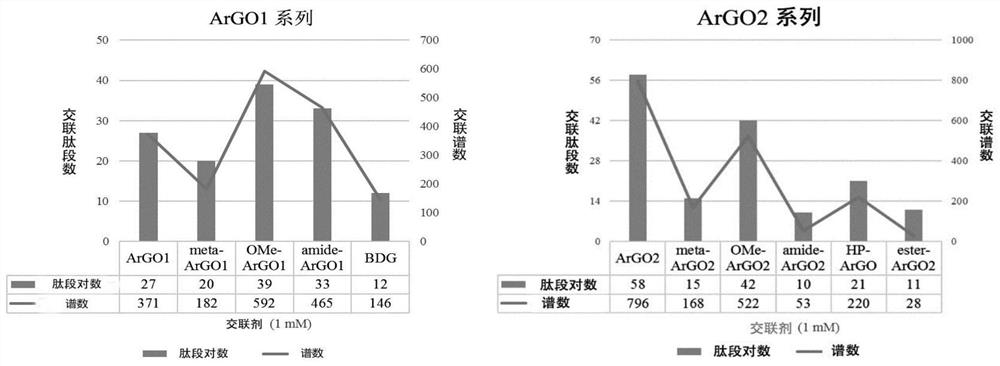
A protein chemical cross-linking agent and its preparation method and application
A chemical cross-linking agent, protein technology, applied in the field of protein and its function research, can solve problems such as poor stability, low cross-linking efficiency, and reduced water-solubility of cross-linking agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The present embodiment adopts the step (1) of the above-mentioned method, carries out the substitution reaction with dibromoethane and p-hydroxyacetophenone as raw materials, and obtains the following product:
[0062]
[0063] The characterization information is as follows:
[0064] White solid; 226mg, yield 76% (calculated with 1mmol dibromoethane and 3mmol p-hydroxyacetophenone as raw materials);
[0065] m.p.167-169°C;
[0066] IR (neat) 1666, 1602, 1253cm -1;
[0067] 1 H NMR (400MHz, CDCl 3 )δ7.95(d, J=8.8Hz, 4H), 6.99(d, J=8.8Hz, 4H), 4.41(s, 4H), 2.56(s, 6H);
[0068] 13 C NMR (100MHz CDCl 3 )δ26.5, 66.6, 114.4, 130.8, 130.9, 162.5, 196.9;
[0069] MS(ESI)m / z 299(M+H) + ;
[0070] HRMS(ESI)m / z C 18 h 19 o 4 (M+H) + The theoretical value is: 299.12779, and the measured value is 299.12764.
[0071] In this example, on the basis of the obtained pre-ArGO-1, the following product is obtained through the reaction of step (2):
[0072]
[0073] Th...
Embodiment 2
[0084] The present embodiment adopts the step (1) of the above-mentioned method, carries out the substitution reaction with diiodoethyl ether and p-hydroxyacetophenone as raw materials, and obtains the following product:
[0085]
[0086] The characterization information is as follows:
[0087] White solid; 278mg, yield 81% (calculated using 1mmol diiodoethyl ether and 3mmol p-hydroxyacetophenone as raw materials);
[0088] m.p.113-115°C;
[0089] IR (neat) 1676, 1601, 1257, 1065cm -1 ;
[0090] 1 H NMR (400MHz, CDCl 3 )δ7.91(d, J=8.8Hz, 4H), 6.93(d, J=8.8Hz, 4H), 4.21(t, J=4.6Hz, 4H), 3.95(t, J=4.7Hz, 4H) ,2.54(s,6H);
[0091] 13 C NMR (100MHz CDCl 3 )δ26.5, 67.7, 69.9, 114.4, 130.6, 130.7, 162.7, 196.9;
[0092] MS(ESI)m / z 343(M+H) + ;
[0093] HRMS(ESI)m / z C 20 h 23 o 5 (M+H) + The theoretical value is 343.15400, and the measured value is 343.15400.
[0094] In this example, on the basis of the obtained pre-ArGO-2, after step (2) reaction, the following p...
Embodiment 3
[0107] In this example, step (1) of the above method is adopted, and 1,2-bis(2-iodoethoxy)ethane and p-hydroxyacetophenone are used as raw materials for substitution reaction to obtain the following product:
[0108]
[0109] The characterization information is as follows:
[0110] White solid; 316mg, yield 82% (calculated using 1mmol 1,2-bis(2-iodoethoxy)ethane and 3 mmol p-hydroxyacetophenone as raw materials);
[0111] m.p.173-175℃;
[0112] IR (neat) 1673, 1600, 1255cm -1 ;
[0113] 1 H NMR (400MHz, CDCl 3 )δ7.91(d, J=8.8Hz, 4H), 6.93(d, J=8.8Hz, 4H), 4.18(t, J=4.7Hz, 4H), 3.89(t, J=4.7Hz, 4H) ,3.77(s,4H),2.54(s,6H);
[0114] 13 C NMR (100MHz CDCl 3 )δ26.5, 67.7, 69.7, 71.1, 114.4, 130.6, 130.7, 162.8, 196.9;
[0115] MS(ESI)m / z 387(M+H) + ,409(M+Na) + ;
[0116] HRMS(ESI)m / z C 20 h 23 o 6 (M+H) + The theoretical value is 387.18022, and the measured value is 387.18094.
[0117] In this example, on the basis of the obtained pre-ArGO-3, the following prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com