Preparation of Prunin and derivative thereof and application of Prunin derivative in drugs for relieving cough and reducing phlegm
A technology of pronin derivative and pronin reaction, applied in the preparation of pronin and its derivatives and its application in cough and phlegm drugs, can solve the problem of low solubility and fat solubility, which limit the application of pronin , Pronin low bioavailability and other issues, to achieve stable quality, good cough and phlegm, rapid curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
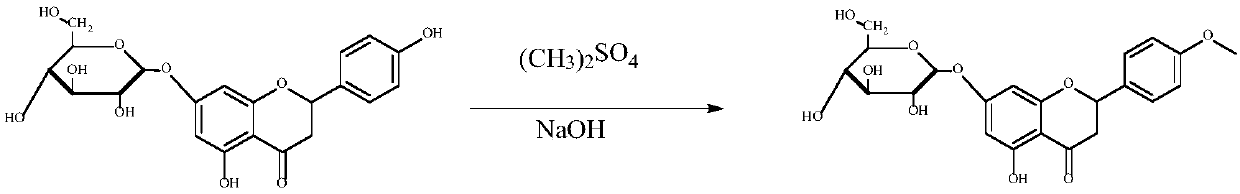
[0023] Preparation Example 1: Take 100 mg of Pronin, add 5 ml of ethanol (95%), and 30 mg of dimethyl sulfate, and then add 15 mg of sodium hydroxide. Stir at room temperature for 5 hours. After the reaction was complete, 20ml of water was added, followed by extraction with ether. The ether layer was dried over anhydrous sodium sulfate. After removing the ether, the crude product was dissolved in 95% ethanol, and purified by column chromatography (silica gel column) to obtain the desired R 3 Prunin compounds whose group is methoxy group (P-3J, it is allowed to contain no more than 10% of R 4 group is methoxy). The chemical structure of the product has passed 1 Identification by H NMR and MS. See the specific reaction figure 1 .
preparation example 2
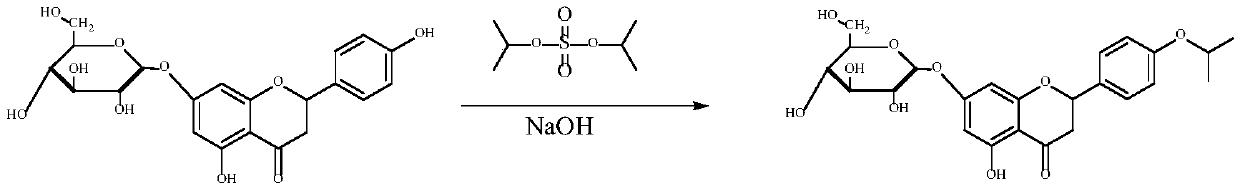
[0024] Preparation example 2: Take 100 mg of Pronin, add 5 ml of isopropanol containing 5% water and 30 mg of diisopropyl sulfate, and then add 15 mg of sodium hydroxide. Stir at room temperature for 2-20 hours. After the reaction was completed, 20ml of water was added, and then extracted with a mixed solvent of petroleum ether: ethyl acetate = 2:1. The organic solvent layer was dried over anhydrous sodium sulfate. After removing the mixed solvent, after the crude product is dissolved in 95% ethanol, use column chromatography (silica gel column) to purify, can obtain required R3 base is the prunin derivative (P-3B, allowing containing not more than 10% of the R4 groups are isopropoxy). The chemical structure of the product has been identified by 1H NMR and MS. See the specific reaction figure 2 .
preparation example 3
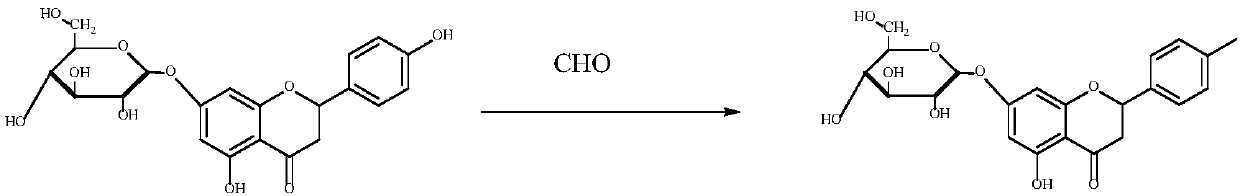
[0025] Preparation Example 3: Take 100 mg of Pronin, add 5 ml of isopropanol containing 5% water and 1 ml of formaldehyde, and then add 15 mg of sodium hydroxide or potassium hydroxide. Stir at room temperature for 1-5 hours. After the reaction was completed, 20 ml of water was added, and then extracted with a mixed solvent of petroleum ether: ethyl acetate = 1:3. The organic solvent layer was dried over anhydrous sodium sulfate. After removing the mixed solvent, the crude product is dissolved in 95% ethanol, and then purified by column chromatography (silica gel column) to obtain the desired R 3 Pronin derivatives whose group is deoxymethyl group (P-3J-1, allowed to contain no more than 10% of R 4The group is deoxymethyl). The chemical structure of the product has been identified by 1HNMR and MS. See the specific reaction image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com