A kind of highly stable lithium-ion battery electrolyte and its preparation method and application
A lithium-ion battery, high-stability technology, used in secondary batteries, circuits, electrical components, etc., can solve the problems of poor cycle performance, increased polarization, and reduced lithium ion diffusion, and achieve improved and stable cycle performance. Lithium ion diffusion and the effect of slowing down the degradation of cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
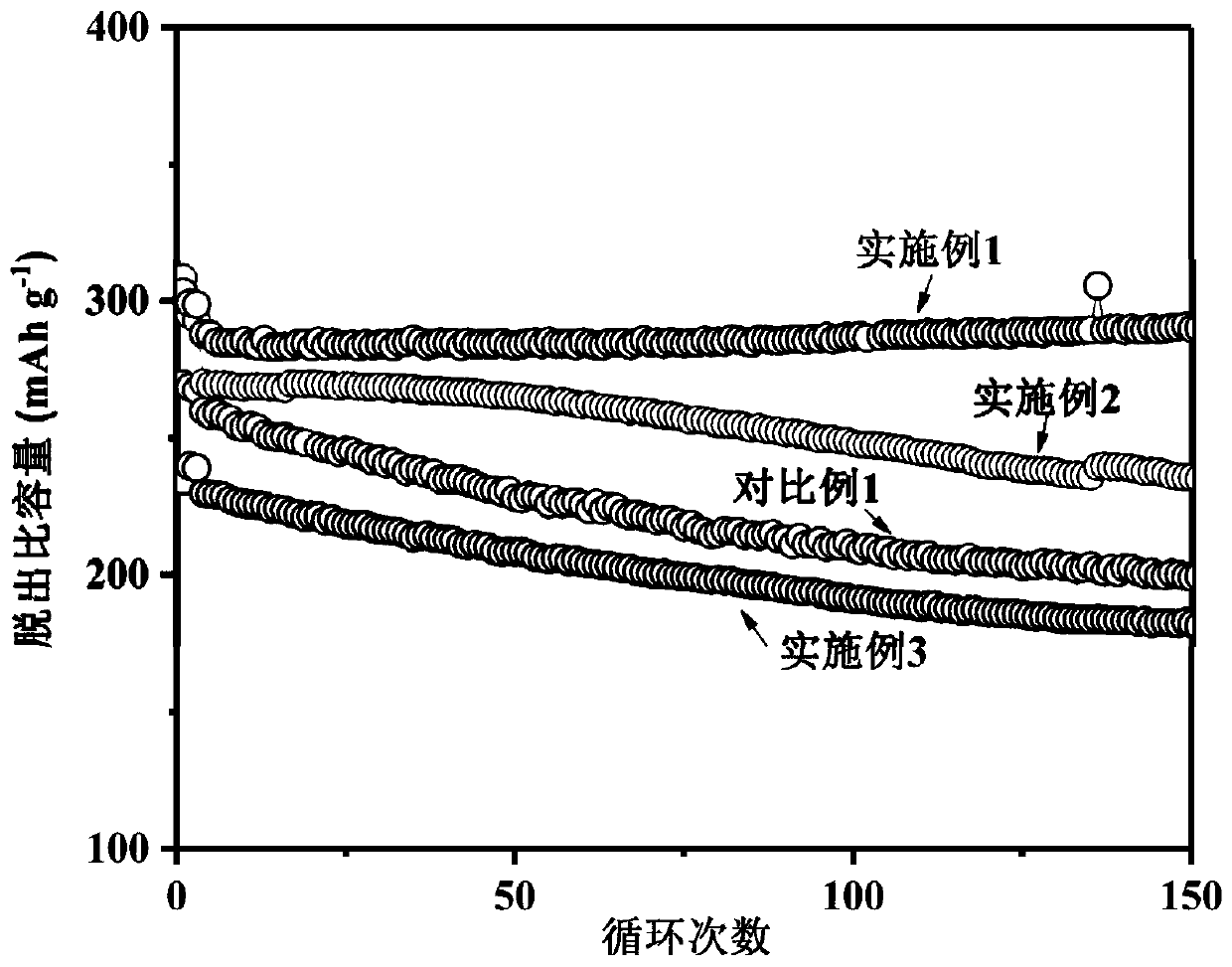
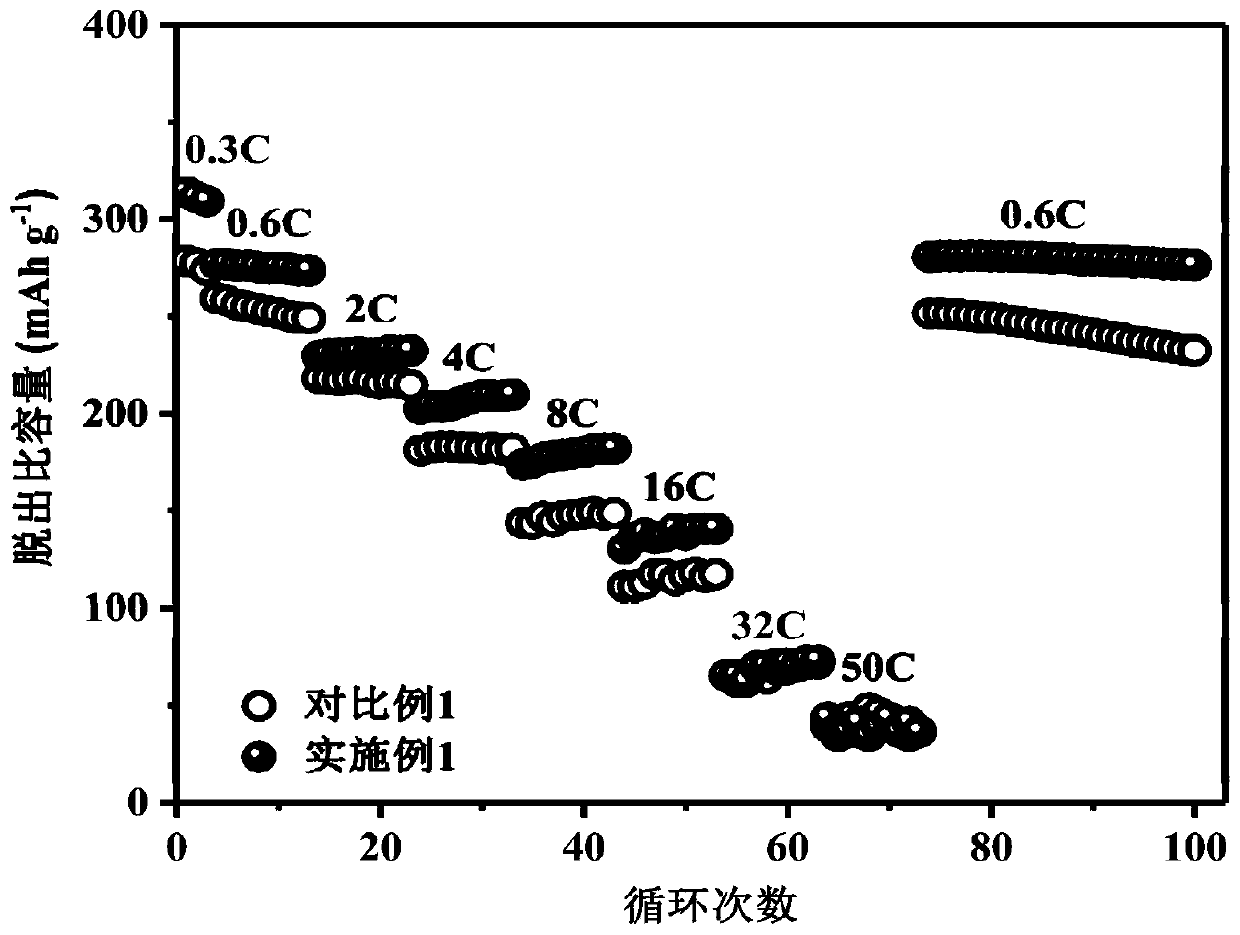
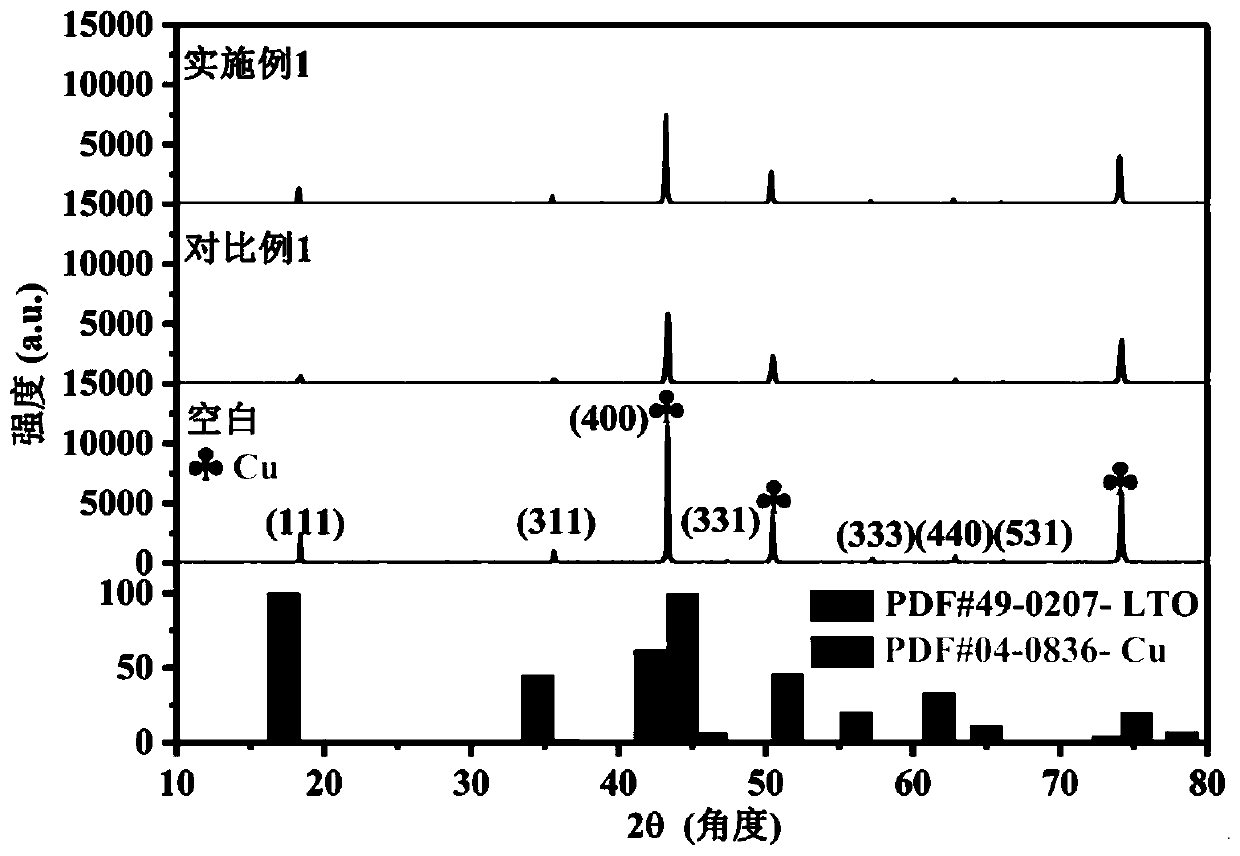
Examples
Embodiment 1
[0030] (1) Mix the cyclic carbonate solvent ethylene carbonate (EC) and the linear carbonate solvent dimethyl carbonate (DMC) according to the mass ratio EC:DMC=1:1, and use molecular sieves, calcium hydride, and lithium hydride to purify Remove impurities and water;
[0031] (2) Dissolve the conductive lithium salt LiTFSI in the solvent obtained in step (1) at room temperature, with a final concentration of 1.0mol / L, and stir evenly to obtain a standard electrolyte;
[0032] (3) The standard electrolyte configuration prepared in step (2) contains Mn[(TFSI)] of 0.3mol / L 2 , to obtain a stable electrolyte for lithium-ion batteries.
Embodiment 2
[0034] (1) Mix the cyclic carbonate solvent ethylene carbonate (EC) and the linear carbonate solvent dimethyl carbonate (DMC) according to the mass ratio EC:DMC=1:1, and use molecular sieves, calcium hydride, and lithium hydride to purify Remove impurities and water;
[0035] (2) Dissolve the conductive lithium salt LiTFSI in the solvent obtained in step (1) at room temperature, with a final concentration of 1.0mol / L, and stir evenly to obtain a standard electrolyte;
[0036] (3) The standard electrolyte configuration prepared in step (2) contains Mn[(TFSI)] of 0.15mol / L 2 , to obtain a stable electrolyte for lithium-ion batteries.
Embodiment 3
[0038] (1) Mix the cyclic carbonate solvent ethylene carbonate (EC) and the linear carbonate solvent dimethyl carbonate (DMC) according to the mass ratio EC:DMC=1:1, and use molecular sieves, calcium hydride, and lithium hydride to purify Remove impurities and water;
[0039] (2) Dissolve the conductive lithium salt LiTFSI in the solvent obtained in step (1) at room temperature to a final concentration of 1.3 mol / L, and stir evenly to obtain a lithium-ion battery electrolyte.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com