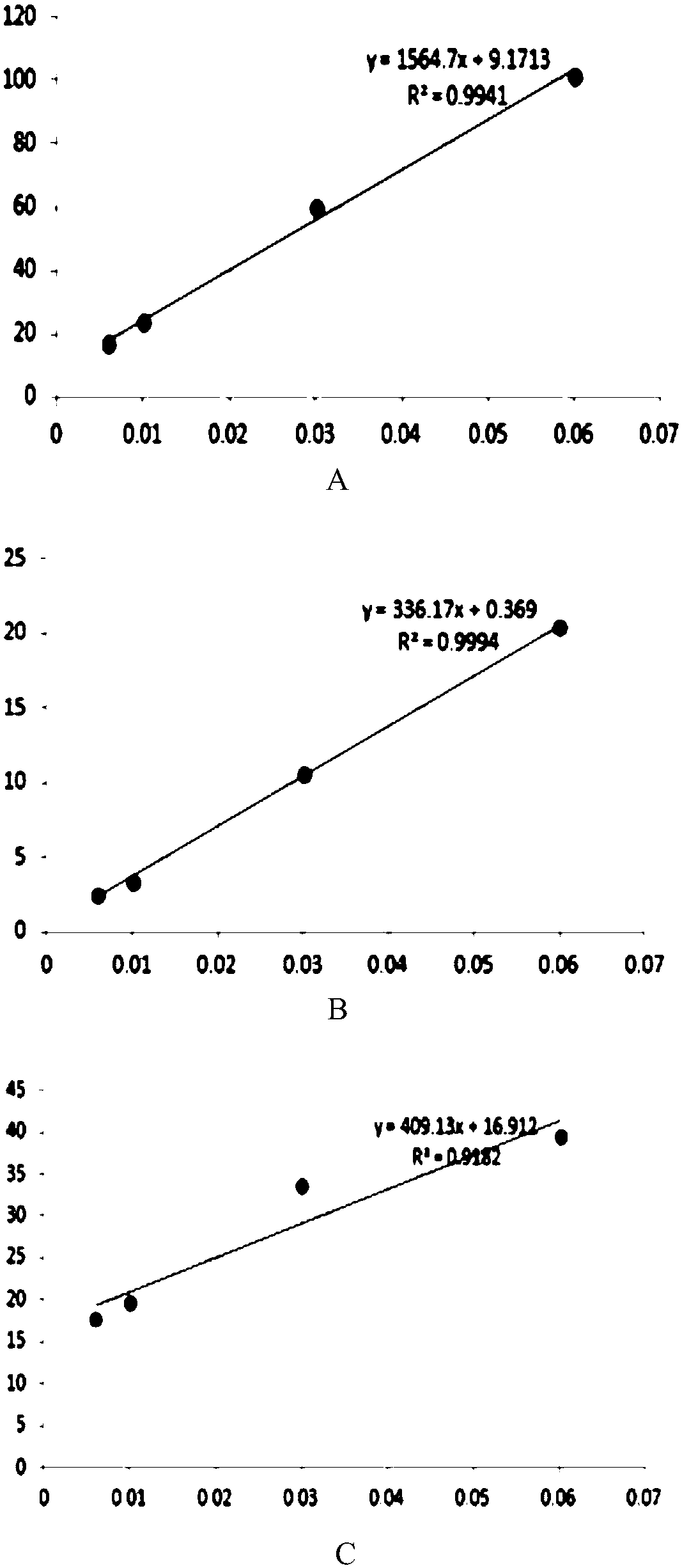
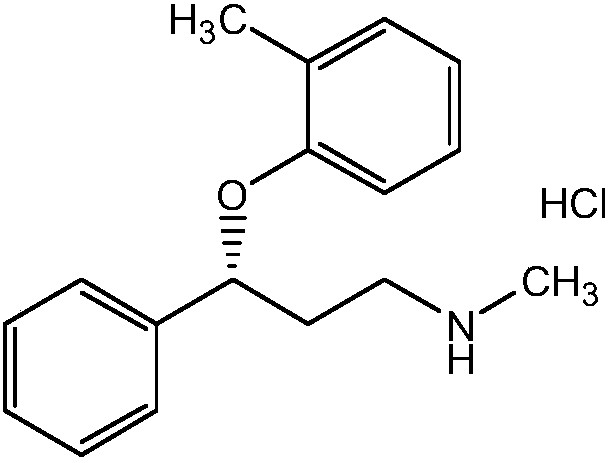
Atomoxetine hydrochloride rapidly disintegrating oral film agent and preparation method thereof
A technology of atomoxetine hydrochloride and oral instant film, which is applied in the direction of medical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc. Affect the medication compliance of patients and children, and achieve the effect of good taste, safe and reliable route of administration, and improvement of medication compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] prescription:
[0035]
[0036] Note: *Used in formulation but removed during processing
[0037] Preparation:
[0038] 1) Add cyclodextrin to purified water at 20-50°C, stir to dissolve, add atomoxetine hydrochloride to the cyclodextrin solution, and stir for 0.5-4 hours to obtain the cyclodextrin-coated atomoxetine hydrochloride Xitine;
[0039]2) Dissolving the film-forming material in water at 50-70°C, adding the atomoxetine hydrochloride cyclodextrin inclusion compound prepared in 1), and continuously stirring;
[0040] 3) Add flavoring agent, plasticizer and disintegrant sequentially to the dispersion formed in 2), and stir for 0.5 hours;
[0041] 4) the drug-containing glue formed in 3) is left to stand for defoaming;
[0042] 5) Coating the drug-containing glue obtained in 4), drying at 50-60° C., and cutting to obtain the atomoxetine hydrochloride film.
Embodiment 2
[0044] prescription:
[0045]
[0046] Note: *Used in formulation but removed during processing
[0047] Preparation:
[0048] 1) Add cyclodextrin to purified water at 20-50°C, stir to dissolve, add atomoxetine hydrochloride to the cyclodextrin solution, and stir for 0.5-4 hours to obtain the cyclodextrin-coated atomoxetine hydrochloride Xitine;
[0049] 2) Dissolving the film-forming material in water at 50-70°C, adding the atomoxetine hydrochloride cyclodextrin inclusion compound prepared in 1), and continuously stirring;
[0050] 3) Add flavoring agent, plasticizer and disintegrant sequentially to the dispersion formed in 2), and stir for 0.5 hours;
[0051] 4) the drug-containing glue formed in 3) is left to stand for defoaming;
[0052] 5) Coating the drug-containing glue obtained in 4), drying at 50-60° C., and cutting to obtain the atomoxetine hydrochloride film.
Embodiment 3
[0054] prescription:
[0055]
[0056] Note: *Used in formulation but removed during processing
[0057] Preparation:
[0058] 1) Add cyclodextrin to purified water at 20-50°C, stir to dissolve, add atomoxetine hydrochloride to the cyclodextrin solution, and stir for 0.5-4 hours to obtain the cyclodextrin-coated atomoxetine hydrochloride Xitine;
[0059] 2) Dissolving the film-forming material in water at 50-70°C, adding the atomoxetine hydrochloride cyclodextrin inclusion compound prepared in 1), and continuously stirring;
[0060] 3) Add flavoring agent, plasticizer and disintegrant sequentially to the dispersion formed in 2), and stir for 0.5 hours;
[0061] 4) the drug-containing glue formed in 3) is left to stand for defoaming;
[0062] 5) Coating the drug-containing glue obtained in 4), drying at 50-60° C., and cutting to obtain the atomoxetine hydrochloride film.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com