Reagents and methods for predicting efficacy of tumor necrosis factor inhibitors
A technology of tumor necrosis factor and inhibitors, applied in the field of reagents for predicting the curative effect of tumor necrosis factor inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
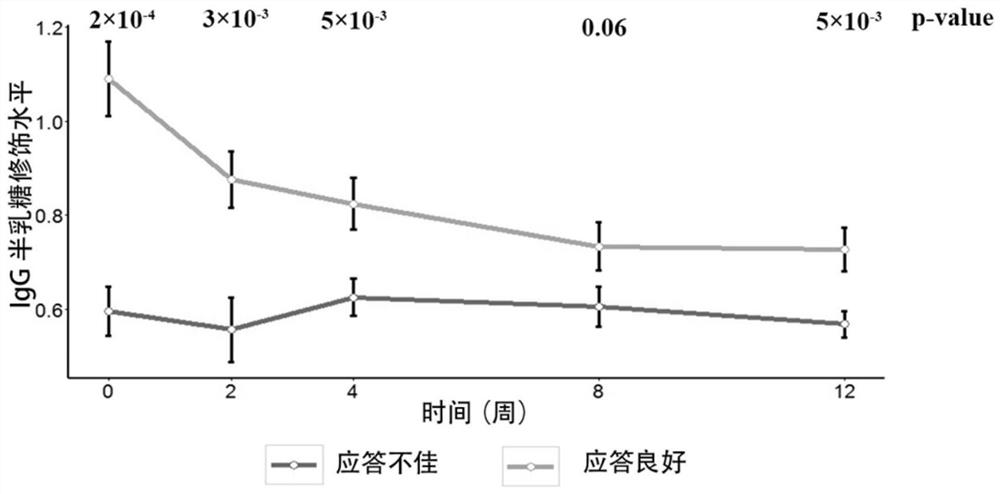
[0136] In this example, a total of 92 patient samples and clinical data were collected, including plasma samples obtained from 79 patients at 0, 2, 4, 8, and 12 weeks after administration to detect IgG galactose modification levels. Wherein the modification level of IgG galactose is carried out according to the method described in CN201510632081.1, including using mass spectrometry to determine the modification level of IgG galactose: G0 (no galactose modification); G1 (one galactose modification), G2 (two galactose modifications) , and quantitatively calculate the IgG galactose modification distribution (IgG-Gal ratio) based on the contents of these three galactose modifications. The specific steps are as follows:
[0137] 1. Purification of IgG from blood samples of screening subjects
[0138] Refer to the Thermo Fisher Scientific Protein A Spin Plate for IgG Screening user manual for implementation, as follows:
[0139] a. Equilibrate the purification column and buffer so...
Embodiment 2
[0168] Whole exome sequencing was performed on 24 responders and 9 nonresponders. Whole exome sequencing uses ultrasound to break up the genome sequence, then uses Roche's capture and library reagents, and then uses illumina Hiseq2000 sequencing. After obtaining the whole exome sequencing data, use Burrows Wheeler Alignment V.0.7.10 for sequence genome alignment, and genome analysis toolkit (GATK) V.1.1.28 for assembly and search for variant sites. Finally, Annovar was used to annotate the variant sites. According to the above process, six candidate gene-related loci were found, including MYOM2, VPS13B, DISP1 and IL27. By expanding the sample and using next-generation sequencing (Sanger) sequencing, it was found that the rs2294066 site of the MYOM2 gene (ie, the 322nd of SEQ ID NO: 3) was significantly different between responding patients and non-responding patients (OR=3.82, p value=1.1×10 -3 )(Table 1).
[0169] Table 1: Distribution of the MYOM2(rs2294066) locus between...
Embodiment 3
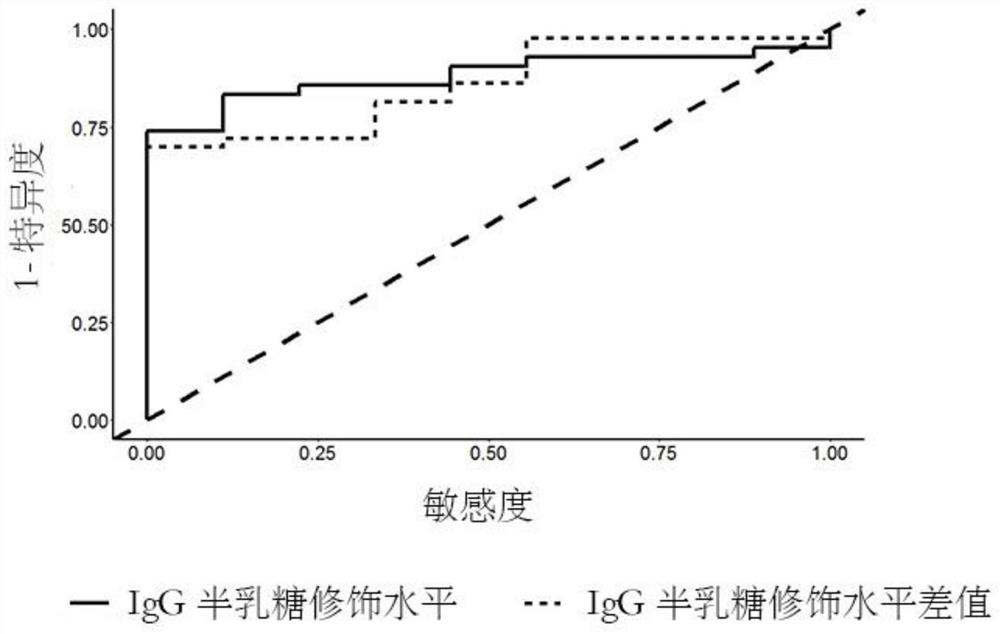
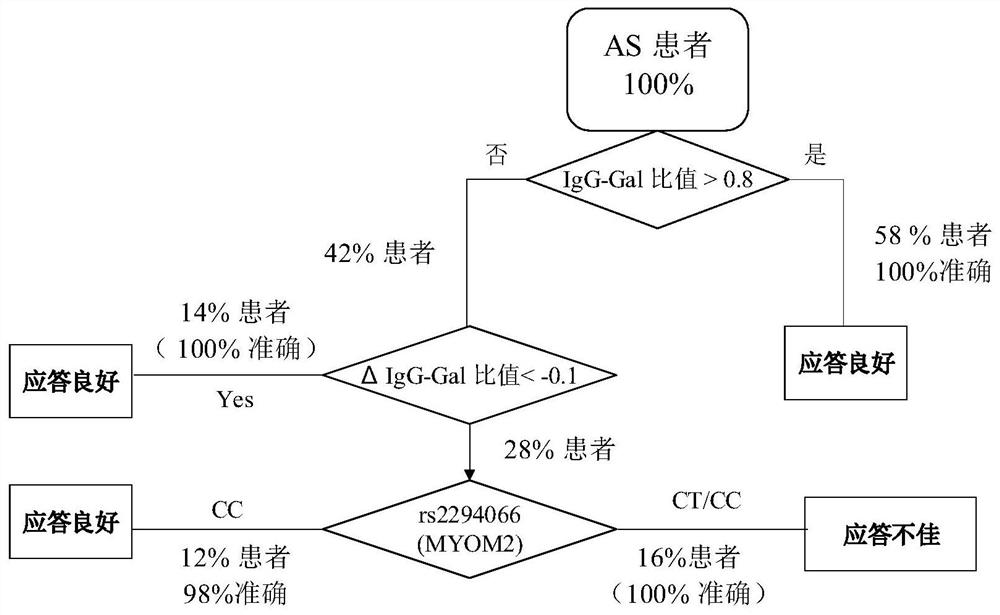
[0177] Since gene loci and IgG galactose modifications represent different genetic backgrounds and transcriptional post-translational modification levels of patients, the present invention predicts the curative effect of tumor necrosis factor antagonist drugs through the combination of the above two biomarkers, thereby assisting clinical more precise treatment.
[0178] according to image 3, when a patient with ankylosing spondylitis considers using tumor necrosis factor inhibitors (fusion protein drugs, such as Yisaipu), first measure the IgG galactose modification level and calculate the Gal ratio, if the Gal ratio is greater than 0.8, then It is predicted that the patient has a good response to the drug, and it is recommended to use the drug for treatment; if the Gal ratio is less than 0.8, it is recommended to use the drug once and measure the IgG galactose modification level again after 2 weeks to calculate the Gal ratio. If the Gal ratio measured after 2 weeks of medic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com