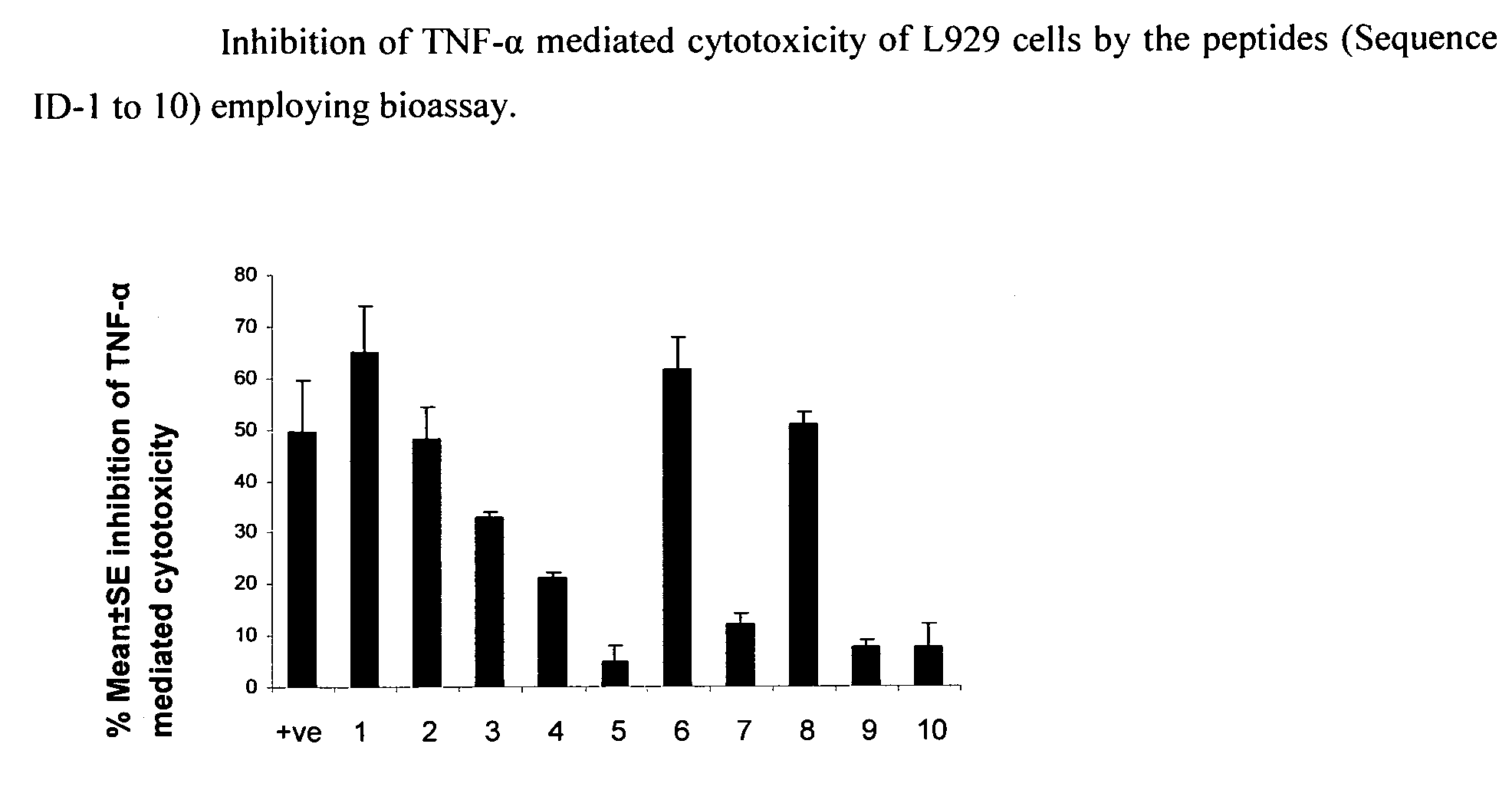Patents
Literature
33 results about "Tumor Necrosis Factor Suppression" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Any therapeutic or preventive procedure that involves inhibition of the production or activity of a Tumor Necrosis Factor. This process may result also in inhibition of some functions of mature immune cells, including polymorphonuclear chemotaxis and monocyte phagocytosis. Tumor Necrosis Factor suppression is intended to favorably modulate functions of the immune system.
Tumor necrosis factor-alpha mutants
The present invention has an object to provide a tumor necrosis factor mutant protein, particularly, a tumor necrosis factor mutant protein specific to TNF-R1 or TNF-R2; tumor necrosis factor inhibitor; or tumor necrosis factor preparation containing it as an effective ingredient, and the object is solved by providing a tumor necrosis factor mutant protein where one or more amino acid residues selected from the group consisting of 29th, 31st, 32nd, 145th, 146th and 147th, or the group consisting of 84th to 89th from the N-terminal of the amino acid sequence of SEQ ID NO:1 is / are replaced with other amino acid residue(s); a tumor necrosis factor inhibitor; and a tumor necrosis factor preparation containing it as an effective ingredient.
Owner:MAYUMI TADANORI +3
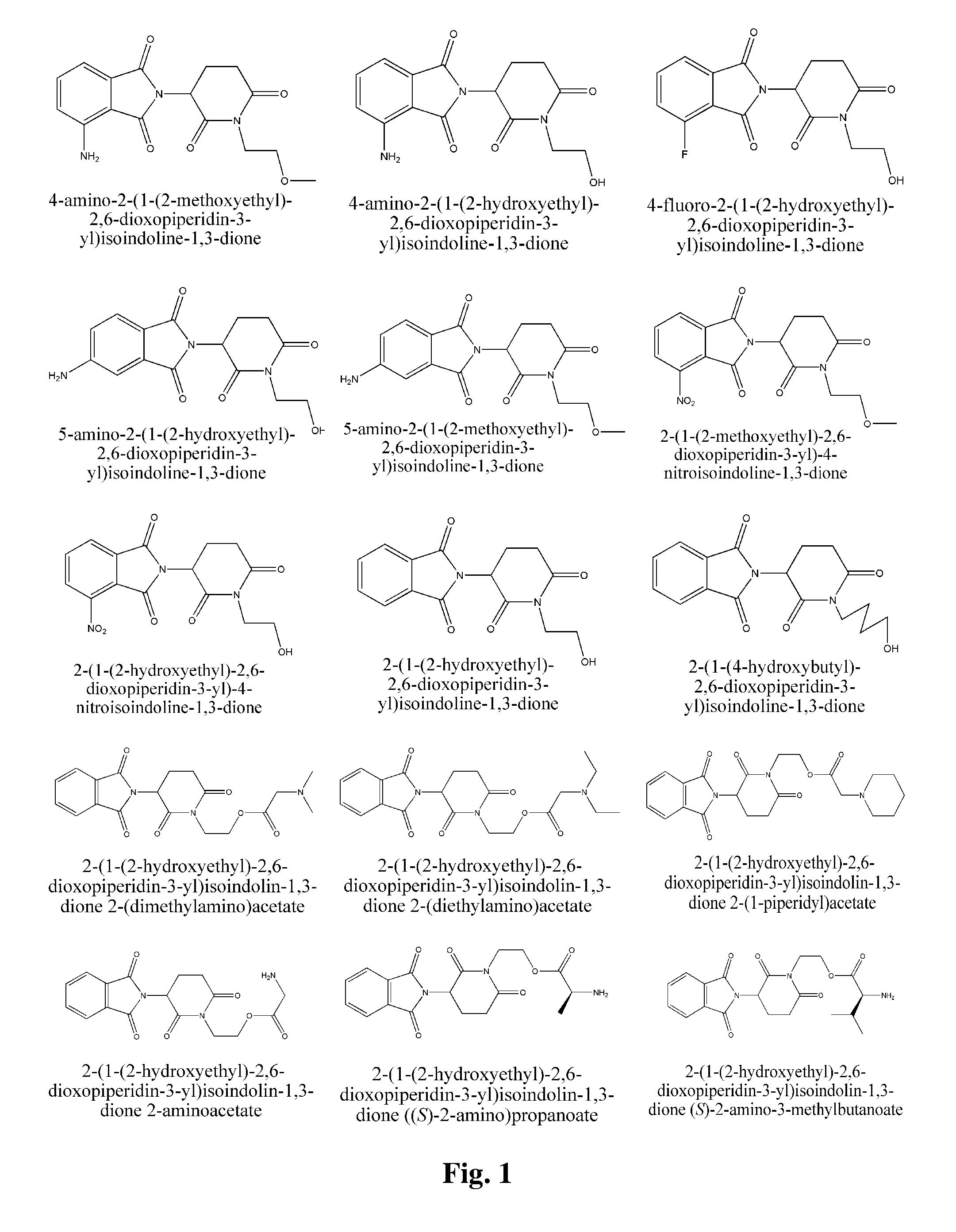
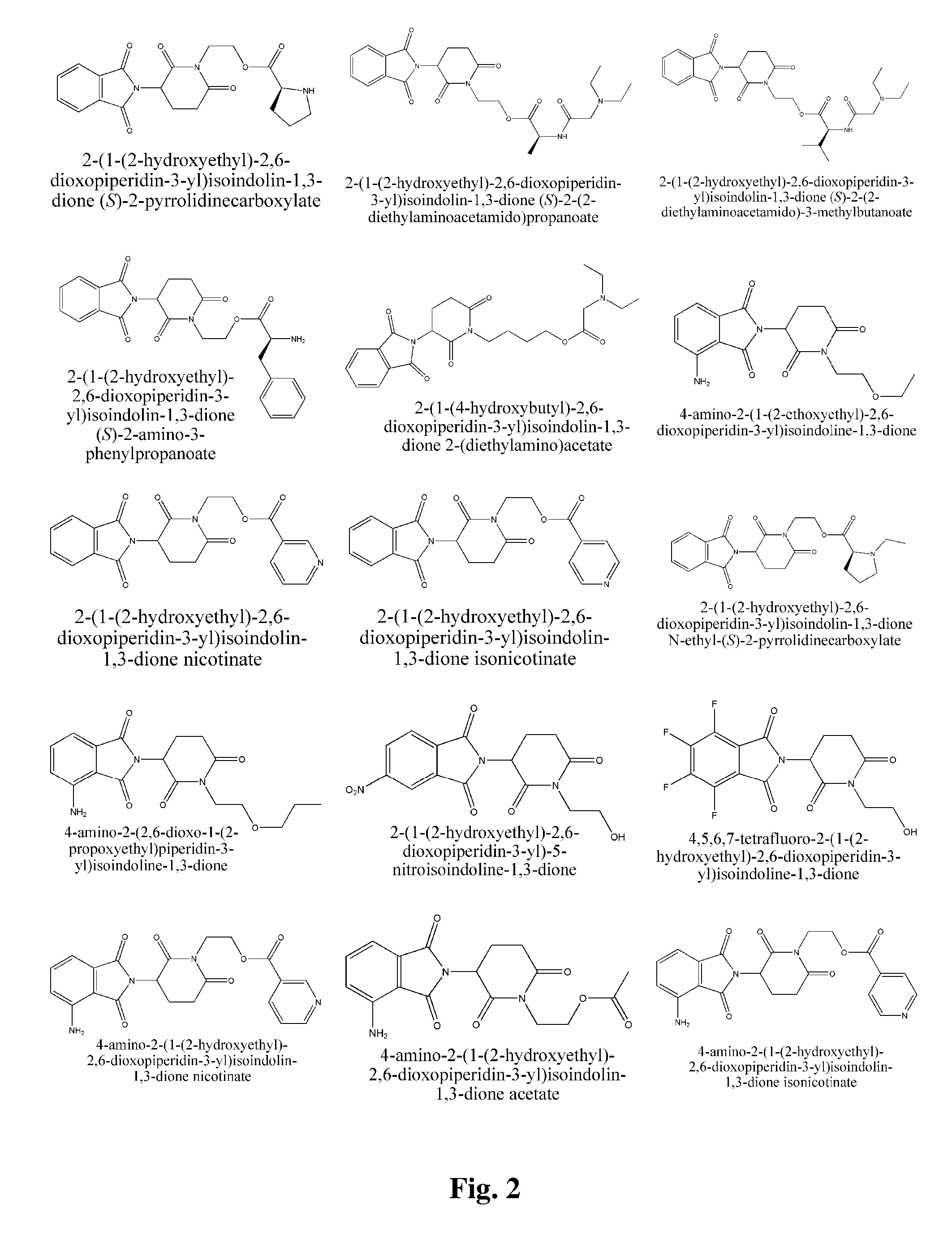
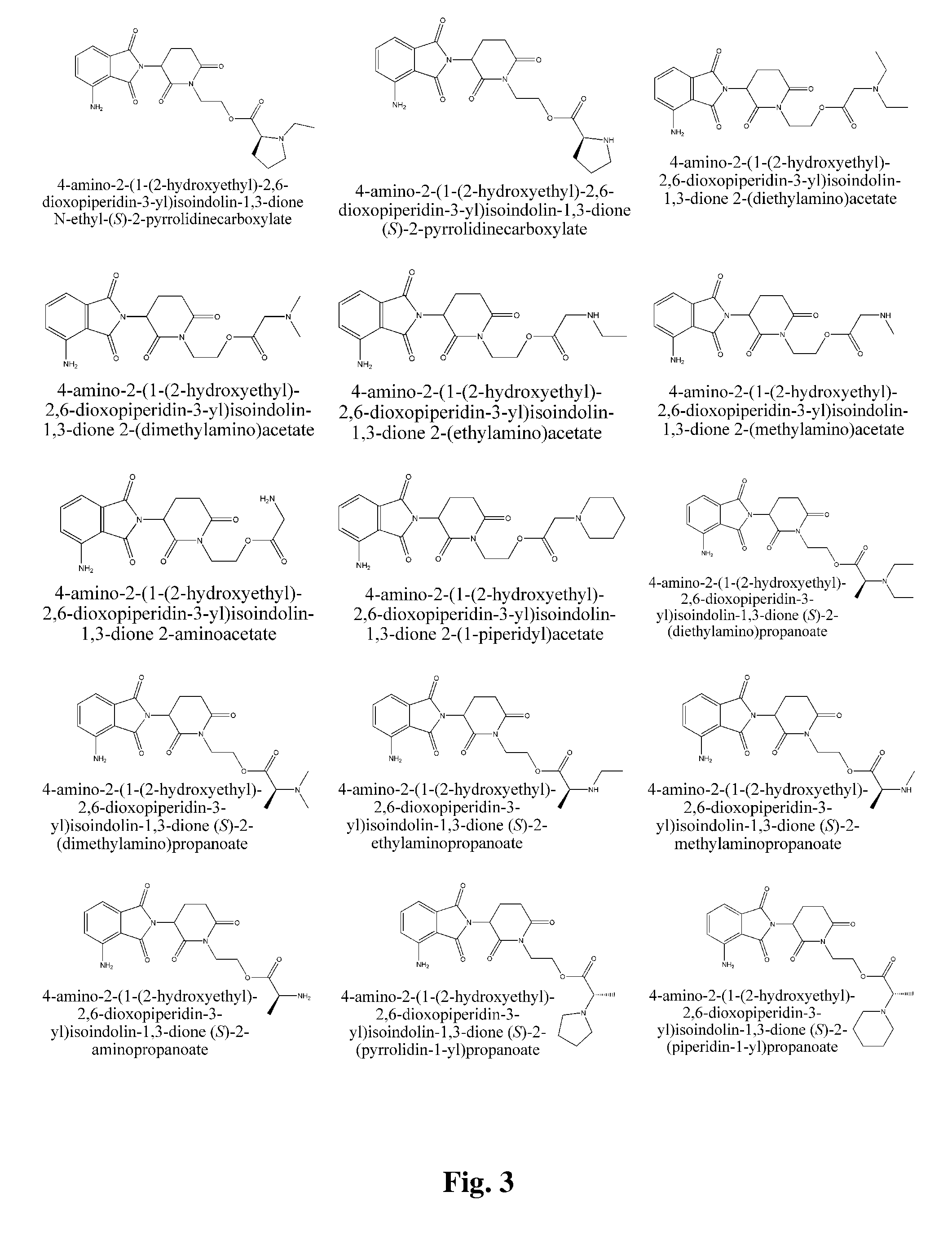
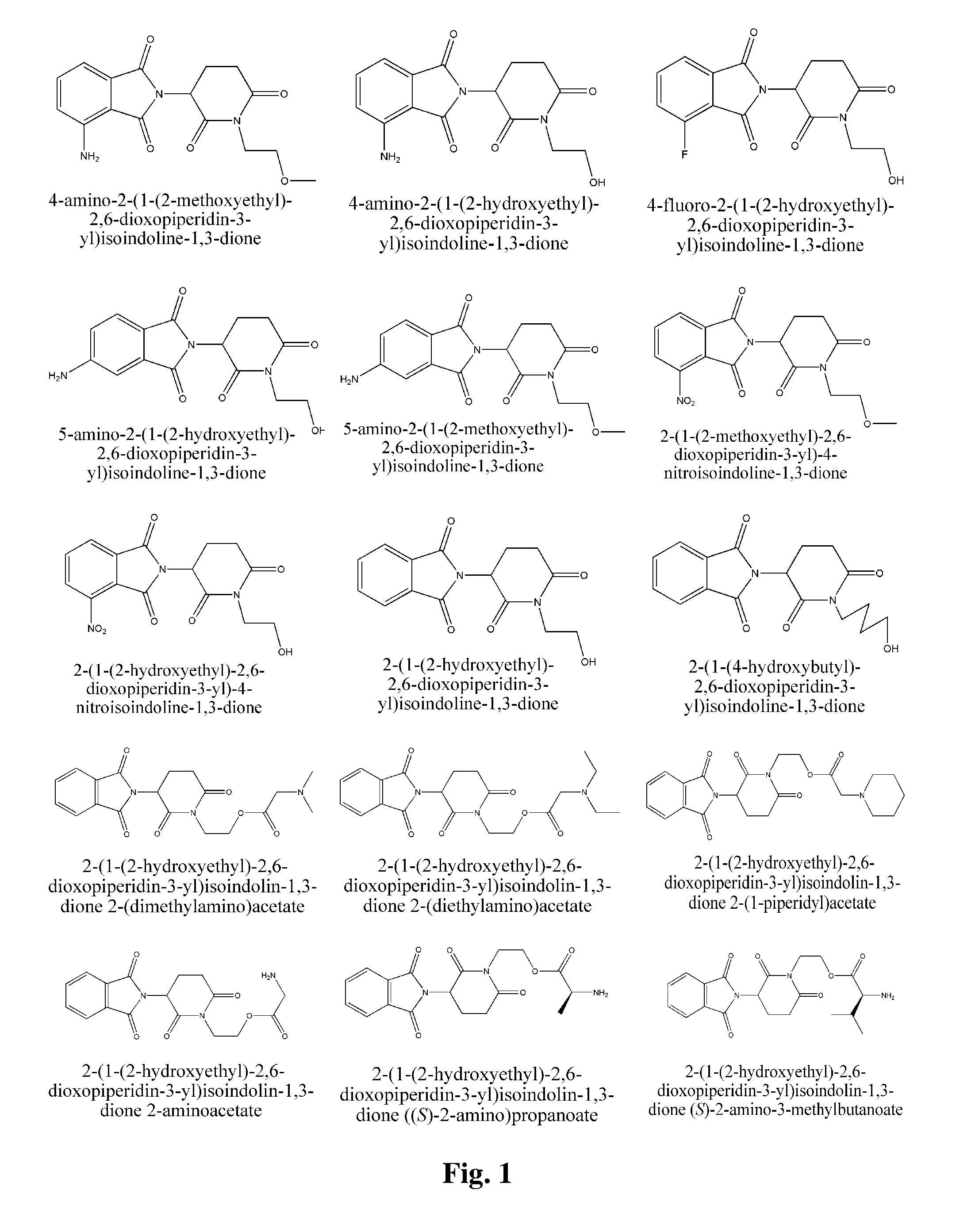
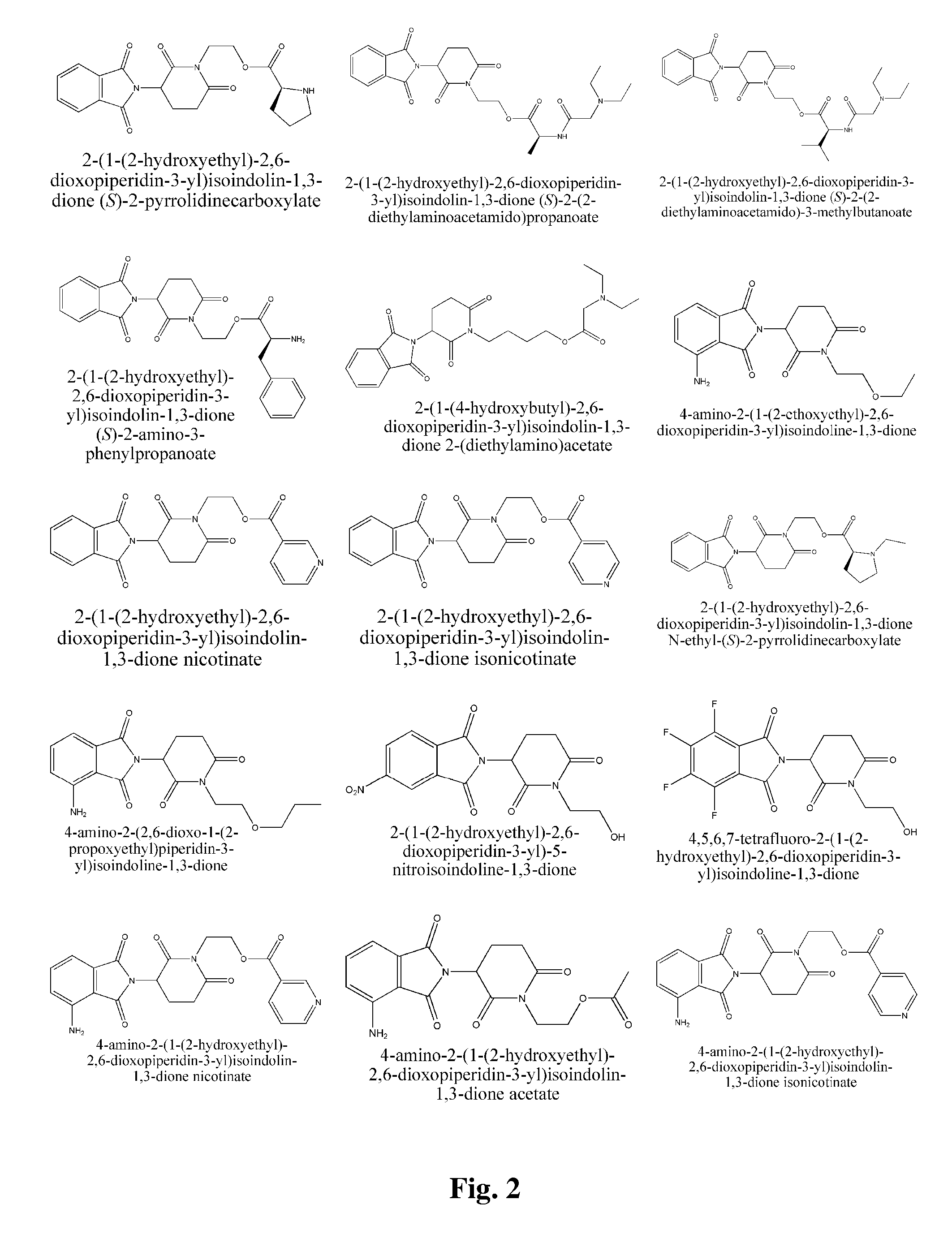
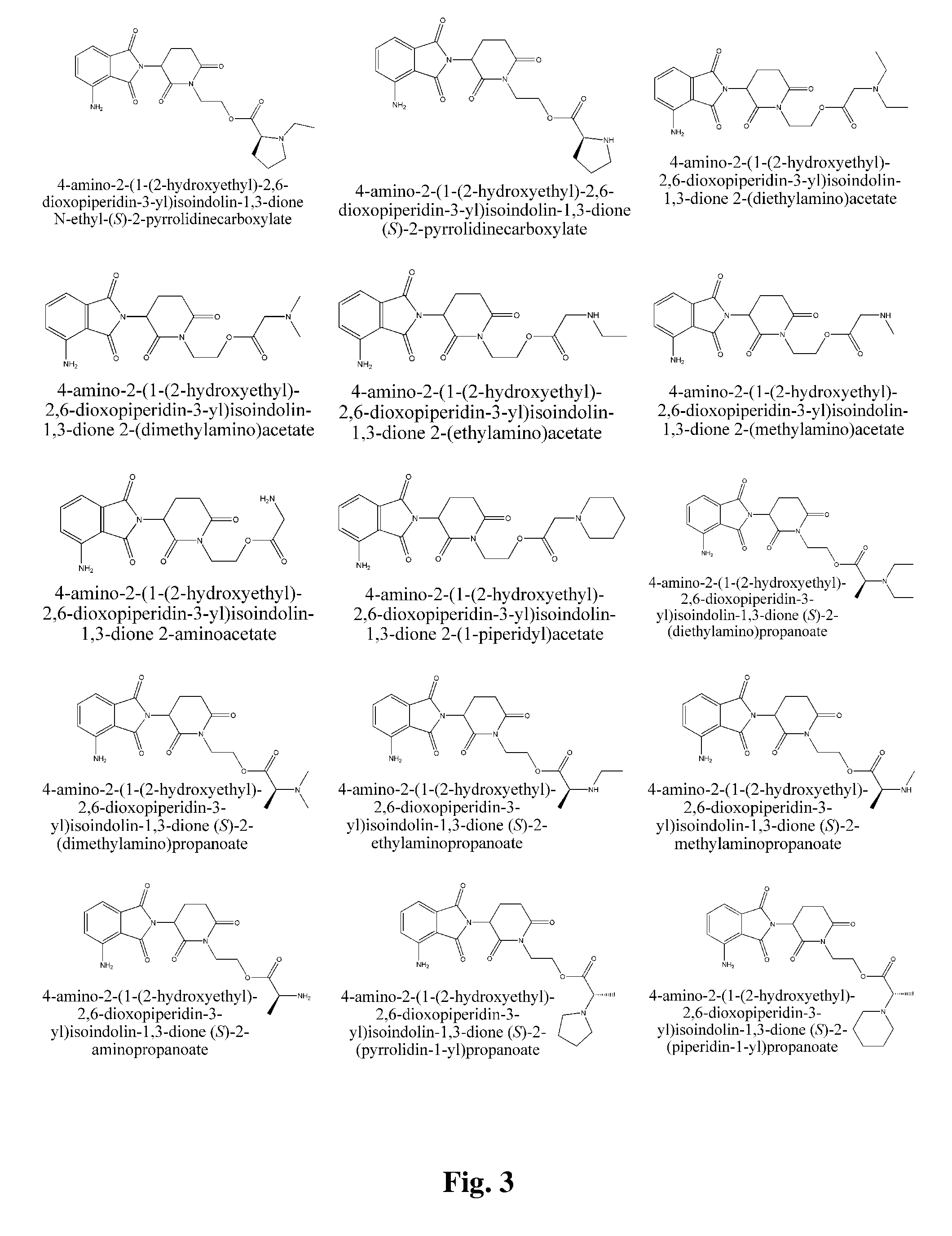
Piperidine-2, 6-dione derivatives and their use as tumor necrosis factor inhibitors
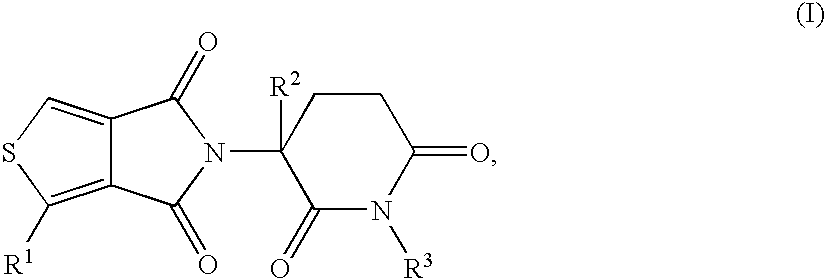
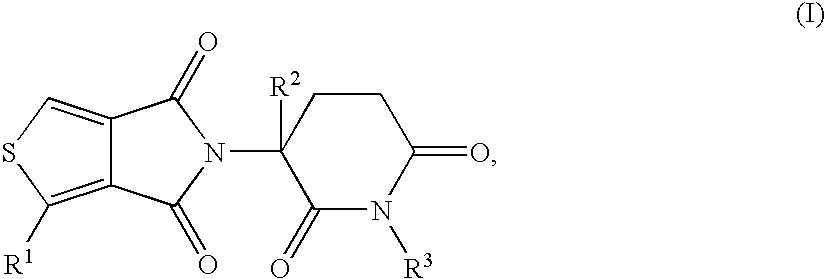
This invention is directed to derivatives of piperidine-2,6-dione, or their organic or inorganic salts thereof, a methods of synthesis of these derivatives, and their application as active pharmaceutical ingredient as inhibitors of TNFα releasing in cells, the derivative of piperidine-2,6-dione being of the general formula (I): wherein n represents 1, 2, 3, 4, 5 or 6; R1 represents from one to four of the same or different substituents selected from F, Cl, Br, C1-4 alkyl, OH, OC1-4 alkyl, NO2, NHC(O)C1-4 alkyl, NH2, NH(C1-4 alkyl), N(C1-4 alkyl)2; R2 represents OR3, NR3R4, N(R3)COR4, O2CR5; R3 and R4 represent independently and at each occurrence H or C1-4 alkyl; R5 represents CHR6NR7R8, CHR6NR9C(O)CHR10NR7R8, a heterocycle W or CHR6NR9C(O)W; R6, R9, R10 represent independently and at each occurrence H, or C1-4 alkyl; R7 and R8 represent independently and at each occurrence H, C1-4 alkyl, or R7 and R8 taken together represent 1,3-propylene, 1,4-butylene, 1,5-pentylene, or 1,6-hexylene; W represents four-membered, five-membered, six-membered, seven-membered, or eight-membered saturated or unsaturated heterocycle.
Owner:TIANJIN HEMAY PHARM SCI TECH CO LTD +1
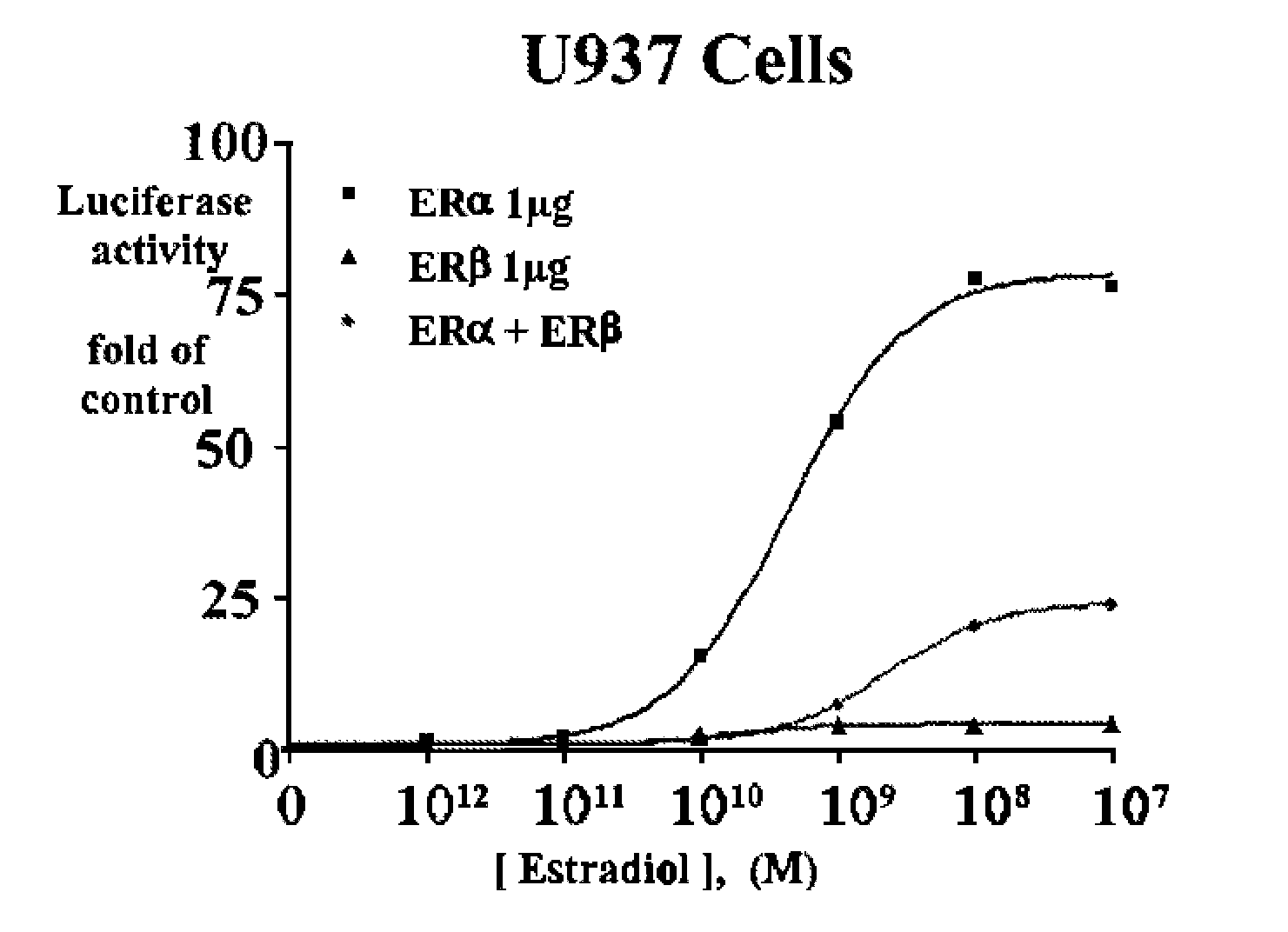
Estrogenic extracts of ligustrum lucidum ait. of the oleaceae family and uses thereof
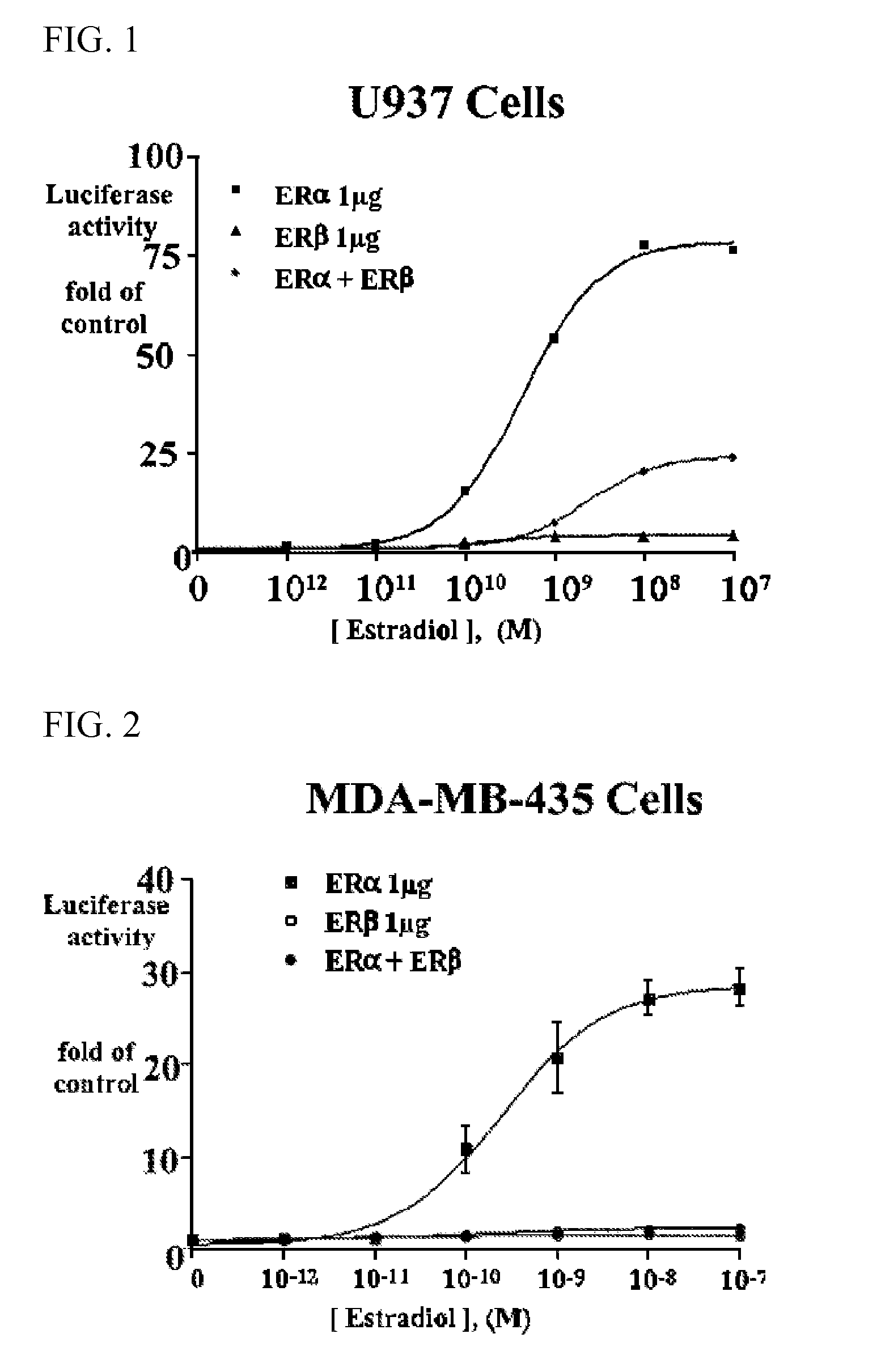
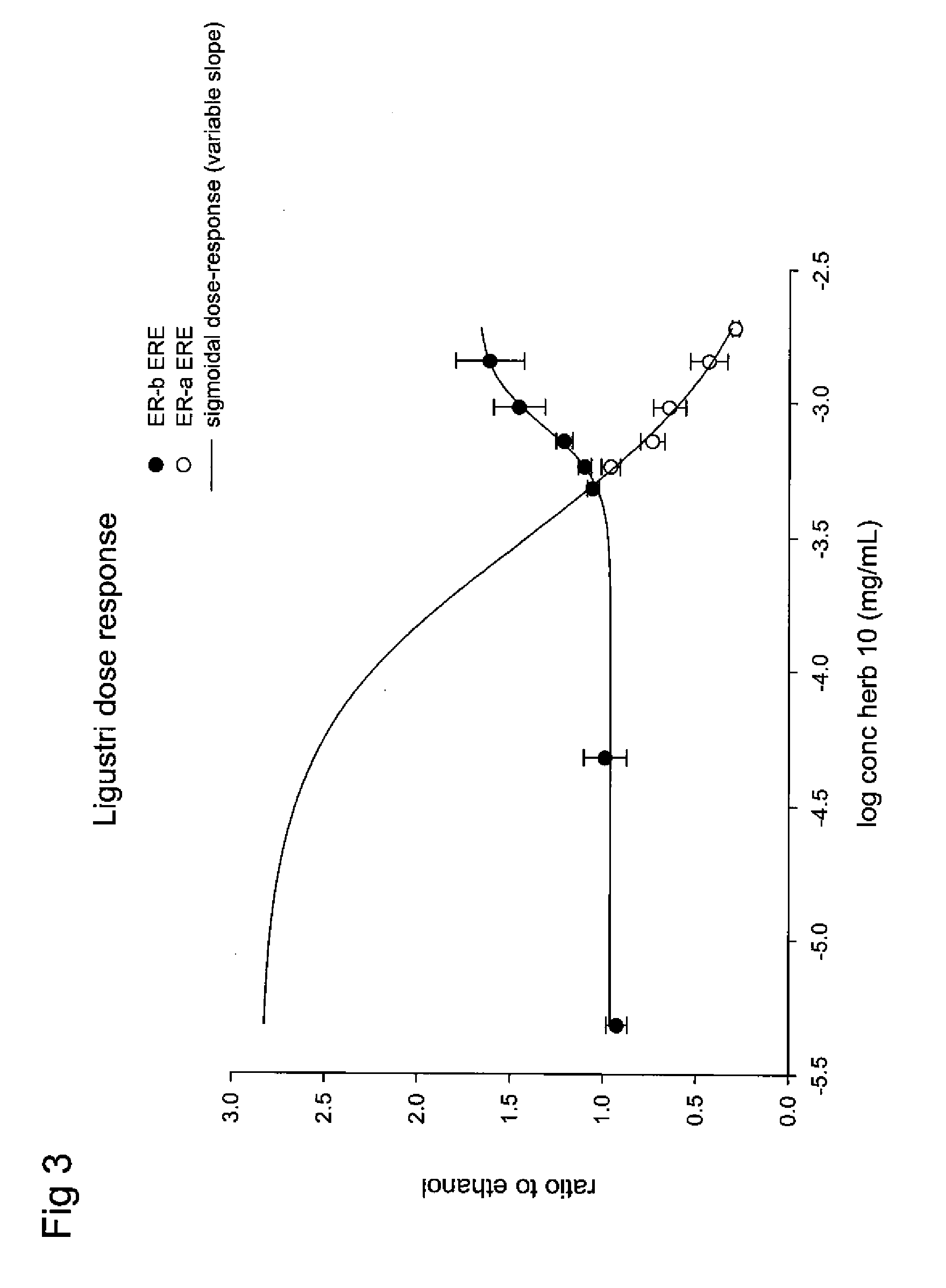
Extracts of various species of the Moraceae family have estrogenic properties. For example, aqueous and ethanolic extracts of Ligustrum lucidum Ait. of the Oleaceae Family possess estrogenic properties in both ERα+ and ERβ+ cells. These estrogenic effects include estrogen response element (ERE) stimulation as well as tumor necrosis factor (TNF) repression. Methods are provided for treating climacteric symptoms, breast and / or uterine cancer, and osteoporosis.
Owner:BIONOVO
Pharmaceutical composition of nanoparticles
InactiveUS8535640B1Sufficient solubilityEnhances intestinal epithelial barrier functionOrganic active ingredientsPowder deliveryNanoparticleSurface charges
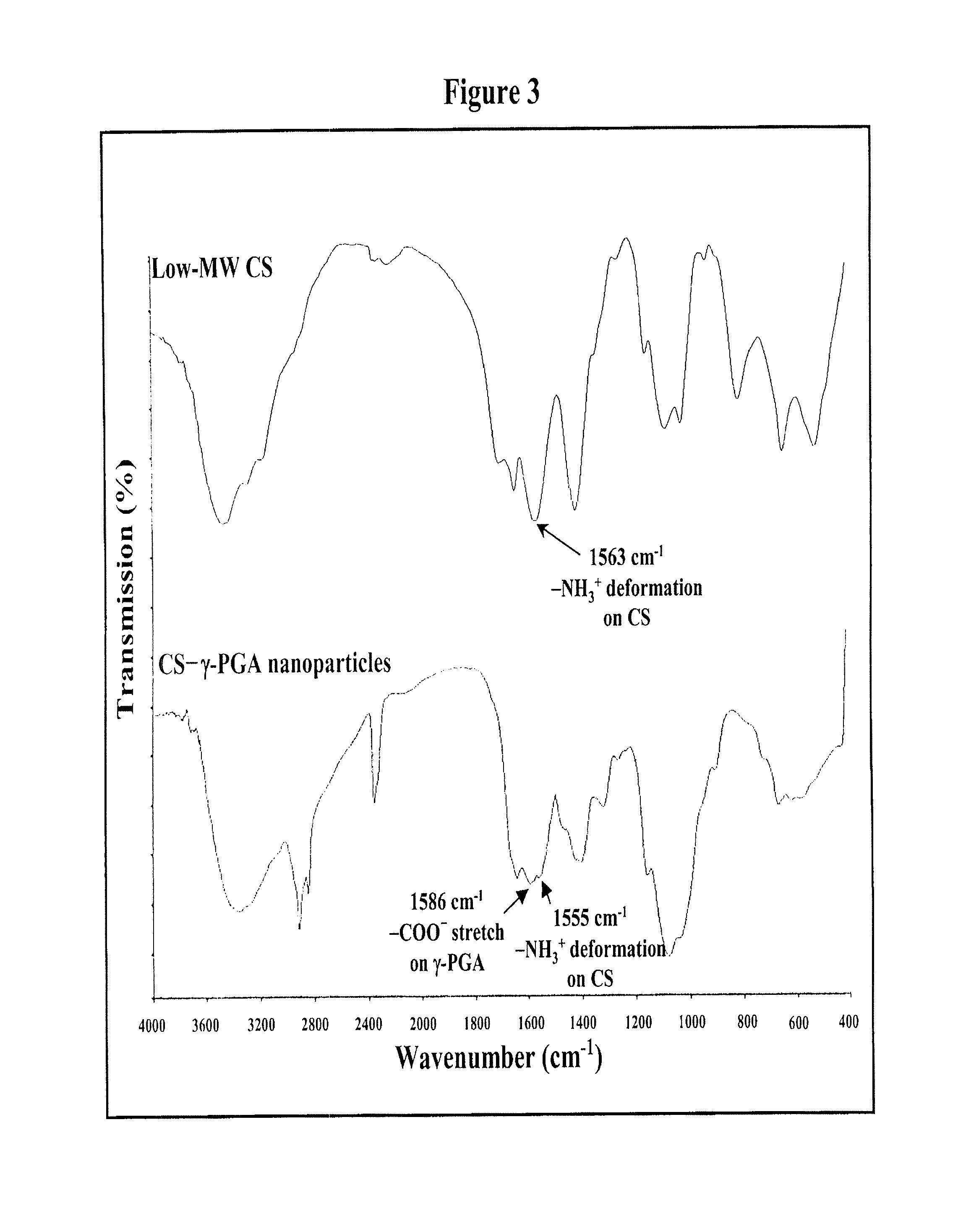
The invention discloses a composition of nanoparticles composed of positively charged chitosan, one negatively charged substrate, and optionally a zero-charge compound or at least one tumor necrosis factor inhibitor for oral delivery. The chitosan-based nanoparticles are characterized with a positive surface charge and enhanced permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Oligonucleotide antagonist for human tumor necrosis factor alpha (TNF-alpha)
InactiveUS7309786B2Strong specificityHigh affinitySugar derivativesPeptide/protein ingredientsL929 cellHigh concentration
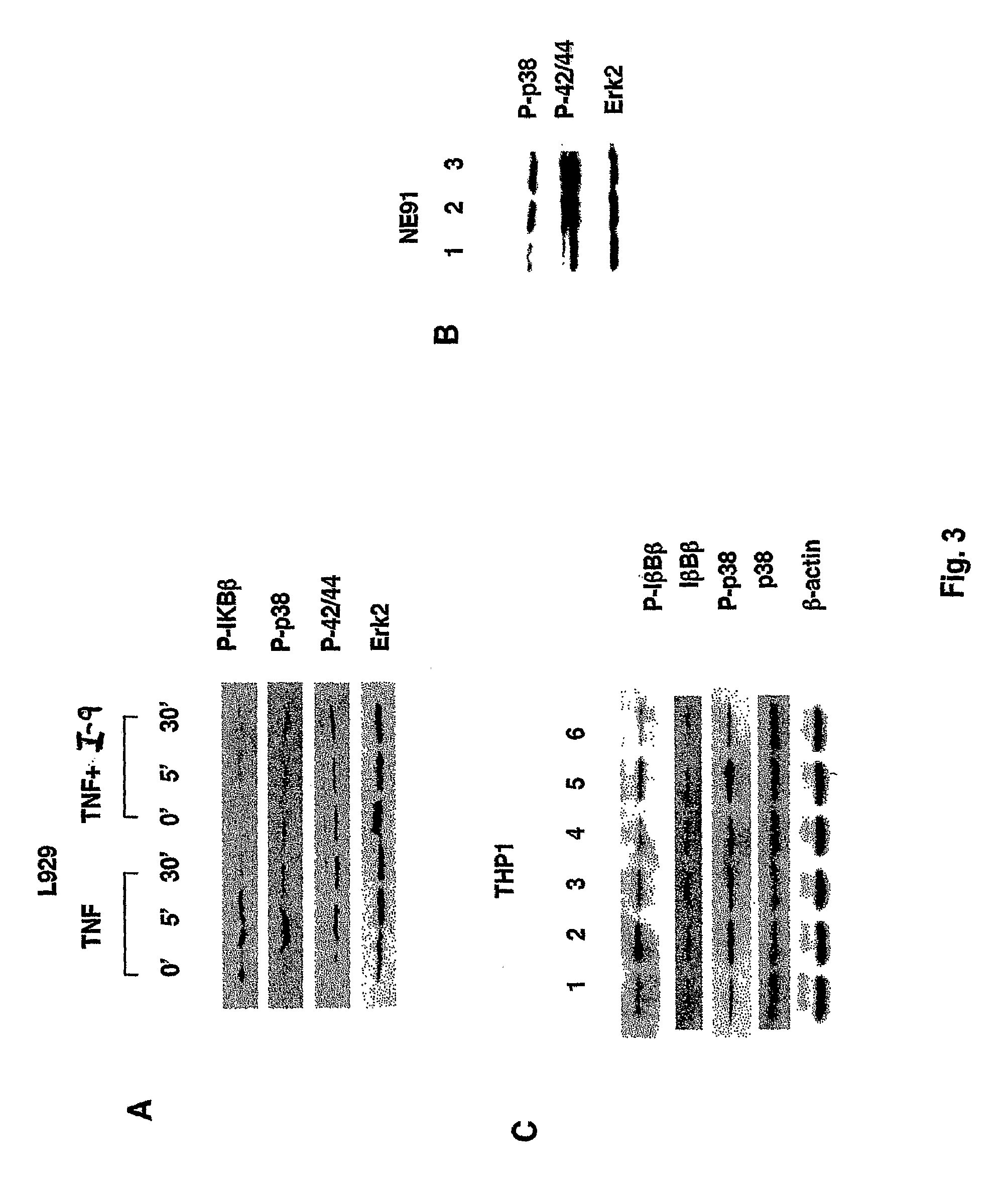
The present invention relates to a group of new oligonucleotides sequences with human tumor necrosis factor α (TNF-α) inhibiting activity, which includes DNA sequences and RNA sequences. These oligonucleotides or aptamer can specifically be bound to TNF-α and inhibit the cytoxicity of TNF-α to L929 cells. Therefore, the aptamer of the present invention may be used to detect TNF-α and provide a therapeutic method for diseases related to the increasing level of TNF-α. Compared with other TNF antagonists such as monoclonal antibody and soluble receptor, the present invention has high specificity, high affinity, quick penetration to target tissue, rapid plasma clearance, and lower immunogenecity. Turthermore, it can be used repeatedly and keeps high concentration in target tissue and the like. It has the advantages of affinity and specificity similar to monoclonal antibodies and also has permeability and pharmacokinetics characteristics similar to small molecular polypeptide. The present invention also refers to derivative of the oligonucleotides sequence, including modified sequence. The present invention may further be manufactured as medicine for therapy and diagnosis of TNF-α related diseases.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Application of substituted aryl hydrazone compound serving as anti-tumor necrosis factor inhibitor medicament
ActiveCN101766602AEffective pharmacological activityOrganic active ingredientsAntineoplastic agentsBone marrow fibrosisAnkylosing spondylitis
The invention relates to clinical application of a substituted aryl hydrazone compound and a derivative thereof serving as an anti-tumor necrosis factor inhibitor. A test result shows that the compound has a lower cytotoxicity in animal bodies, so the medical compound is expected to be used as an anti-TNF-alpha and TNF-beta inhibitor in the clinical application and is mainly used for treating some TNF related immunological diseases such as rheumatic arthritis, inflammatory bowel diseases, diabetes, hematosepsis, psoriasis, ankylosing spondylitis and some communicable diseases including HIV. A current TNF related research also shows that the medical compound can be used for treating clinical blood and solid tumor such as myelodysplastic syndrome, myelofibrosis, acute bone marrow cell leucocythemia, acute / chronic graft-versus-host reaction, ovarian cancer, nephrocyte cancer and the like.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
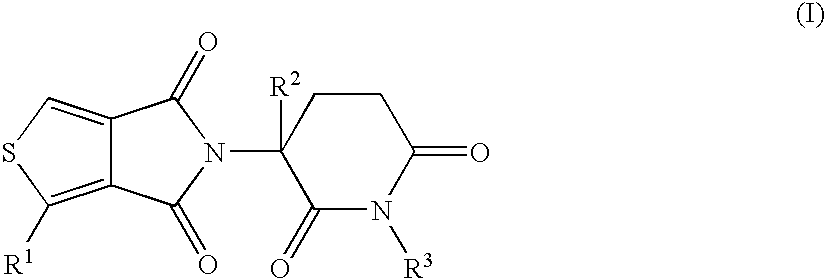
5H-THIOENO(3,4-c)PYRROLE-4,6-DIONE DERIVATIVES AND THEIR USE AS TUMOR NECROSIS FACTOR INHIBITORS
Taught are derivatives of 5H-thioeno(3,4-c)pyrrole-4,6-dione, their organic, and inorganic salts, methods of synthesis of these derivatives, and their application as active pharmaceutical-ingredients useful as inhibitors of TNFα, the derivative being represented by the general formula (I):in which R1 represents H, C1-6alkyl, OR4, OC(O)R5, NO2, NHC(O)R6, or NR7R8; R2 represents H, a halogen, or C1-6alkyl; R3 represents H, methyl, isopropyl, allyl, benzyl, CH2CO2(C1-6alkyl), or CH2(CH2)nR9; R4, R5, R6, R7, and R8 each independently and at each occurrence represents H, or C1-6alkyl; R9 represents H, C1-6alkyl, OH, OC1-6alkyl, NH2, NHC1-6alkyl, N(C1-6alkyl)2, or CO2(C1-6alkyl); and n represents 1, 2, 3, or 4.
Owner:TIANJIN HEMAY BIO TECH CO LTD
DNA encoding tumor necrosis factor inhibitory protein and its use
InactiveUS20050245731A1Antagonize effectReduced cytotoxic activityPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsADAMTS ProteinsCytotoxicity
Recombinant DNA encoding Tumor Necrosis Factor (TNF) Inhibitory Protein, or an active fragment thereof, is obtained. The TNF Inhibitory Protein has the ability to inhibit: (a) the binding of TNF to its receptors, and (b) the cytotoxic effect of TNF. TNF Inhibitory Protein and salts, functional derivatives and active fractions thereof can be used to antagonize the deleterious effects of TNF by eliminating TNF from the body, and to reduce the cytotoxic activity of TNF by binding to TNF and thereby to inhibit the binding of TNF to its receptors. The DNA may be in an expression vector. Host cells transfected with such an expression vector may be used to produce the TNF Inhibitory Protein.
Owner:YEDA RES & DEV CO LTD
5H-thioeno(3,4-c)pyrrole-4,6-dione derivatives and their use as tumor necrosis factor inhibitors
Owner:TIANJIN HEMAY BIO TECH CO LTD
Piperidine-2, 6-dione derivatives and their use as tumor necrosis factor inhibitors
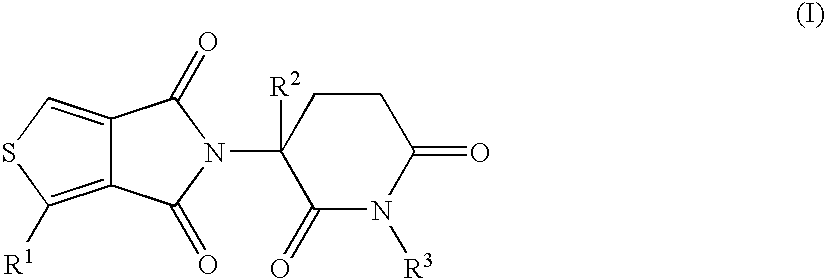
This invention is directed to derivatives of piperidine-2,6-dione, or their organic or inorganic salts thereof, a methods of synthesis of these derivatives, and their application as active pharmaceutical ingredient as inhibitors of TNFα releasing in cells, the derivative of piperidine-2,6-dione being of the general formula (I):wherein n represents 1, 2, 3, 4, 5 or 6; R1 represents from one to four of the same or different substituents selected from F, Cl, Br, C1-4 alkyl, OH, OC1-4 alkyl, NO2, NHC(O)C1-4 alkyl, NH2, NH(C1-4 alkyl), N(C1-4 alkyl)2; R2 represents OR3, NR3R4, N(R3)COR4, O2CR5; R3 and R4 represent independently and at each occurrence H or C1-4 alkyl; R5 represents CHR6NR7R8, CHR6NR9C(O)CHR10NR7R8, a heterocycle W or CHR6NR9C(O)W; R6, R9, R10 represent independently and at each occurrence H, or C1-4 alkyl; R7 and R8 represent independently and at each occurrence H, C1-4 alkyl, or R7 and R8 taken together represent 1,3-propylene, 1,4-butylene, 1,5-pentylene, or 1,6-hexylene; W represents four-membered, five-membered, six-membered, seven-membered, or eight-membered saturated or unsaturated heterocycle.
Owner:TIANJIN HEMAY PHARM SCI TECH CO LTD +1
Application of japonicone A in preparation of tumor necrosis factor (TNF) inhibitor drugs
InactiveCN102406637AHigh clinical application valueOrganic active ingredientsSkeletal disorderDiseaseStructural formula
The invention provides an application of japonicone A (chemical structural formula I) in preparation of tumor necrosis factor (TNF) inhibitor drugs. The japonicone A is inula japonica extract. In the invention, by adopting a reverse docking technology for virtual screening, the result shows that TNF-alpha is potential binding protein of the japonicone A; by adopting a BIACORE technology, the result shows that the japonicone A can be directly combined with TNF-alpha coupled on a chip, and can inhibit the combination of the TNF-alpha and the TNF-R (receptor) coupled on a sensor chip; and furthermore, an experiment confirms that the japonicone A can be directly combined with the TNF-alpha and can inhibit the interaction of the TNF-alpha and the TNF-R, thus the japonicone A can be used for preparing TNF inhibitor drugs. The drug provided by the invention is a pharmaceutical composition prepared from a medicinal carrier and the japonicone A used as an active ingredient. The pharmaceutical composition can be used for treating TNF-related diseases such as osteoarthropathy, asthma, bronchitis, allergic rhinitis and the like, thereby providing a new way for treating the diseases and obtaining greater clinical application values.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Tumor necrosis factor inhibitors
The present invention is directed to compounds that are allosteric inhibitors of tumor necrosis factor receptor I, compositions comprising such compounds, and methods of using such compounds and compositions thereof in the treatment of TNF-α mediated conditions.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Reagent and method for predicting curative effect of tumor necrosin inhibitor
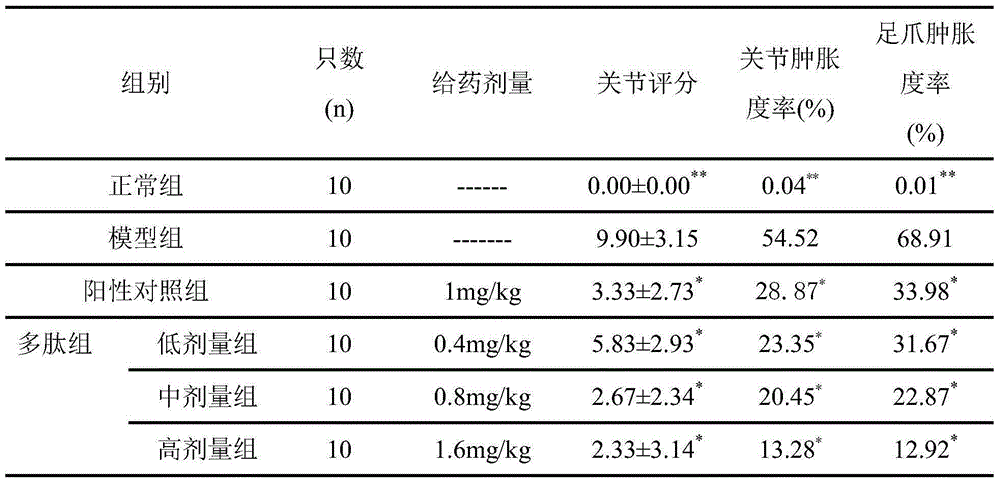
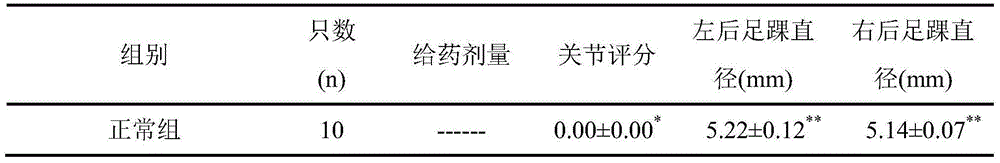
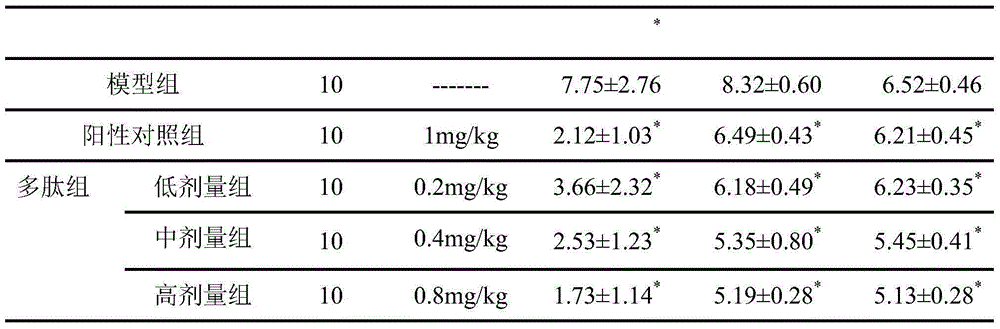
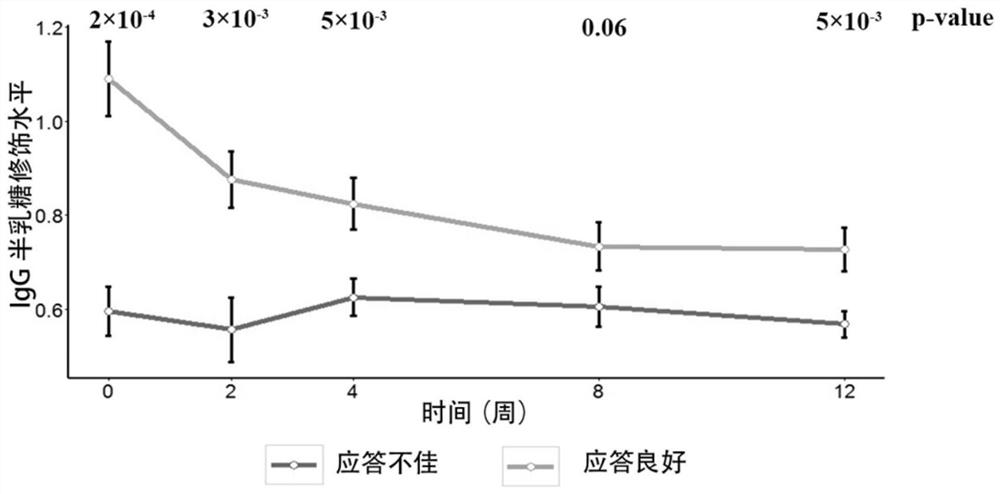
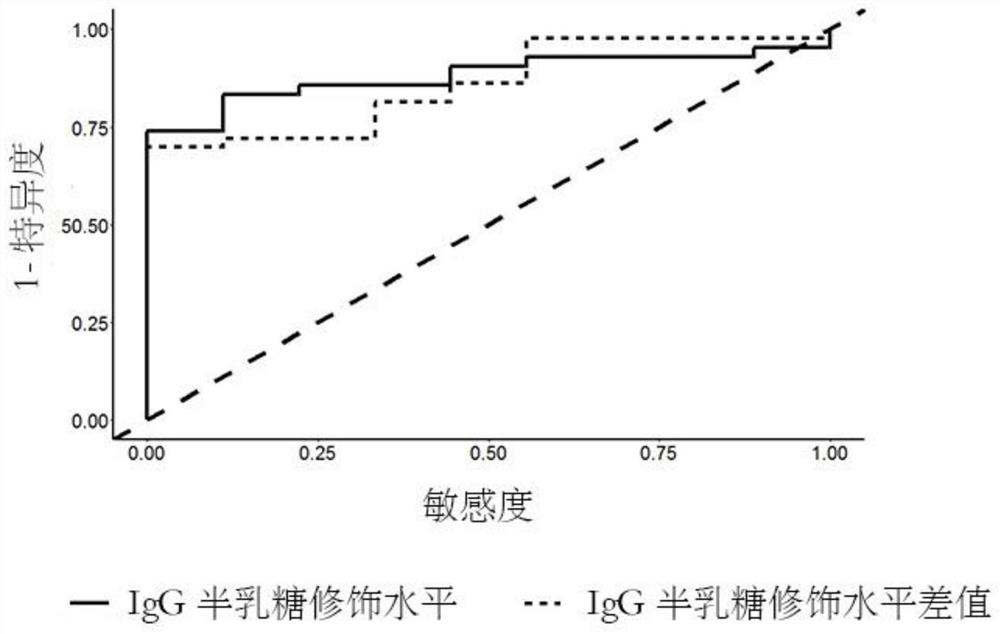
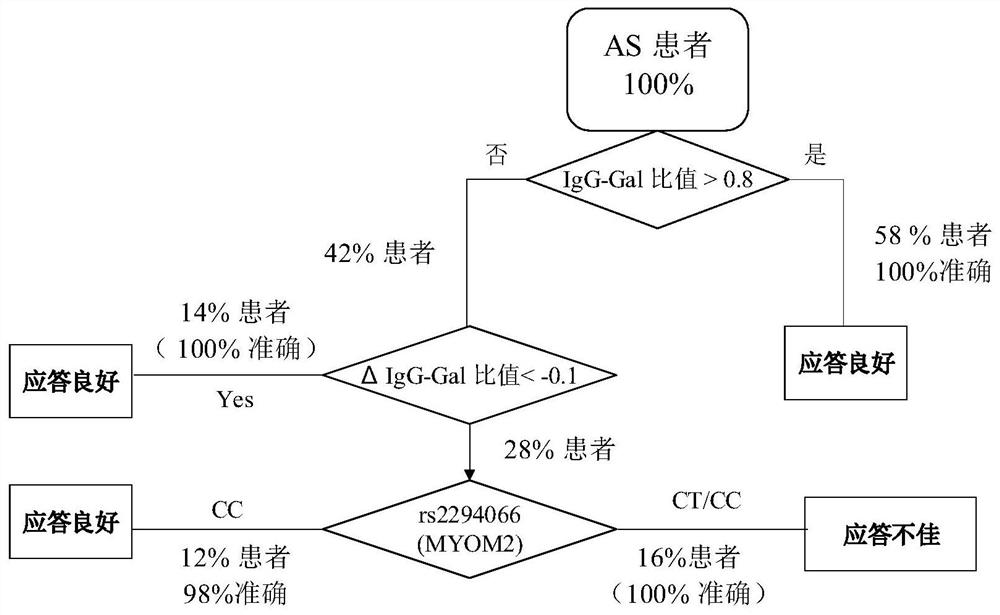
ActiveCN107817353AEasy to answerComponent separationMaterial analysis by electric/magnetic meansCurative effectAnkylosing spondylitis
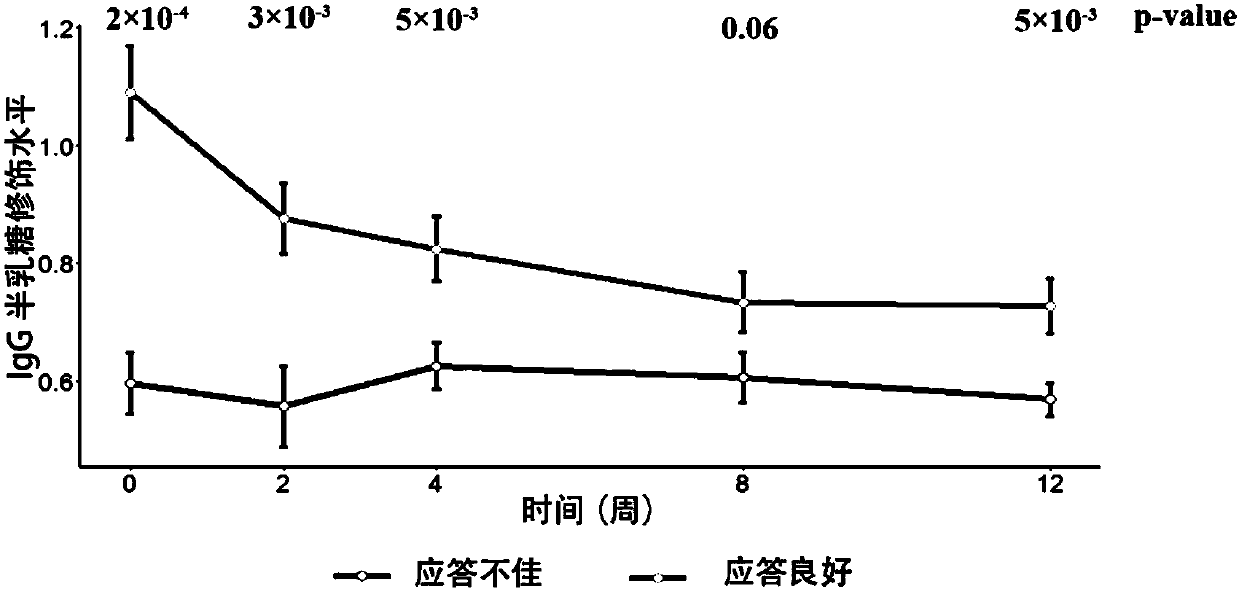
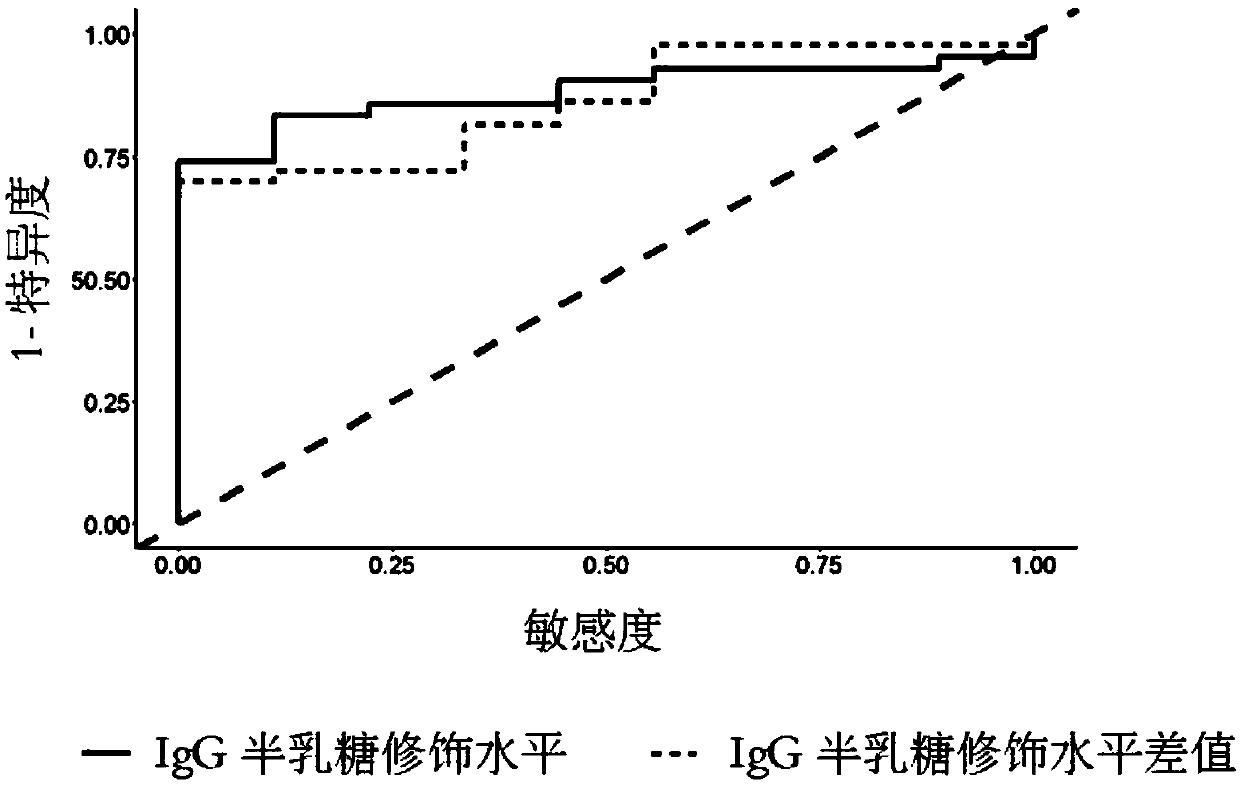
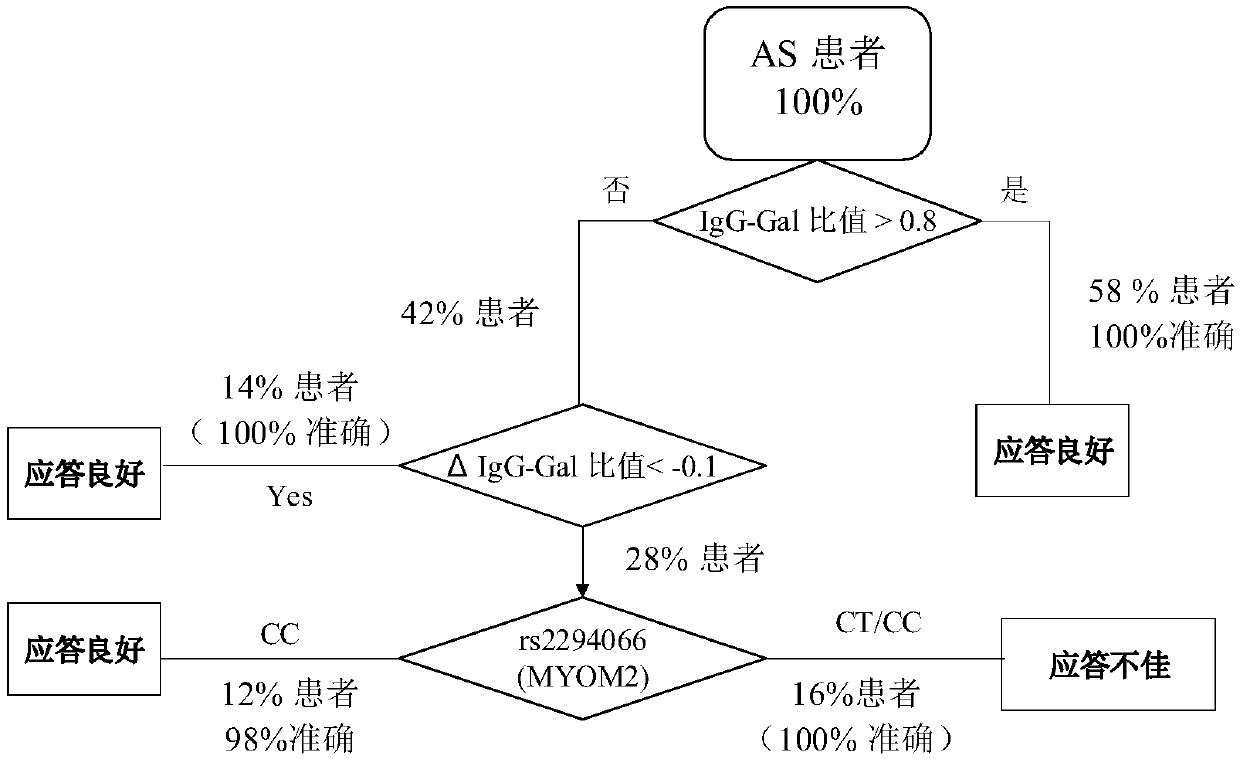
The invention relates to a reagent and a method for predicting the curative effect of the tumor necrosin inhibitor. Particularly, the invention provides application of a reagent and / or an instrument to the preparation of a product for predicting the curative effects of treating patients with ankylosing spondylitis by using tumor necrosin inhibitors, wherein the reagent and / or instrument comprisesa tail end galactosylation level of the blood IgG surface double-antenna complicated N sugar chain, wherein the tail end galactosylation level is double-antenna complicated N-sugar chain level Ga10 connected with no galactose at the tail end, a double-antenna complicated N-sugar chain level Gal1 connected with one galactose at the tail end and a double-antenna complicated N-sugar chain level Gal2connected with two galactoses at the tail end on the IgG surface.
Owner:FUDAN UNIV
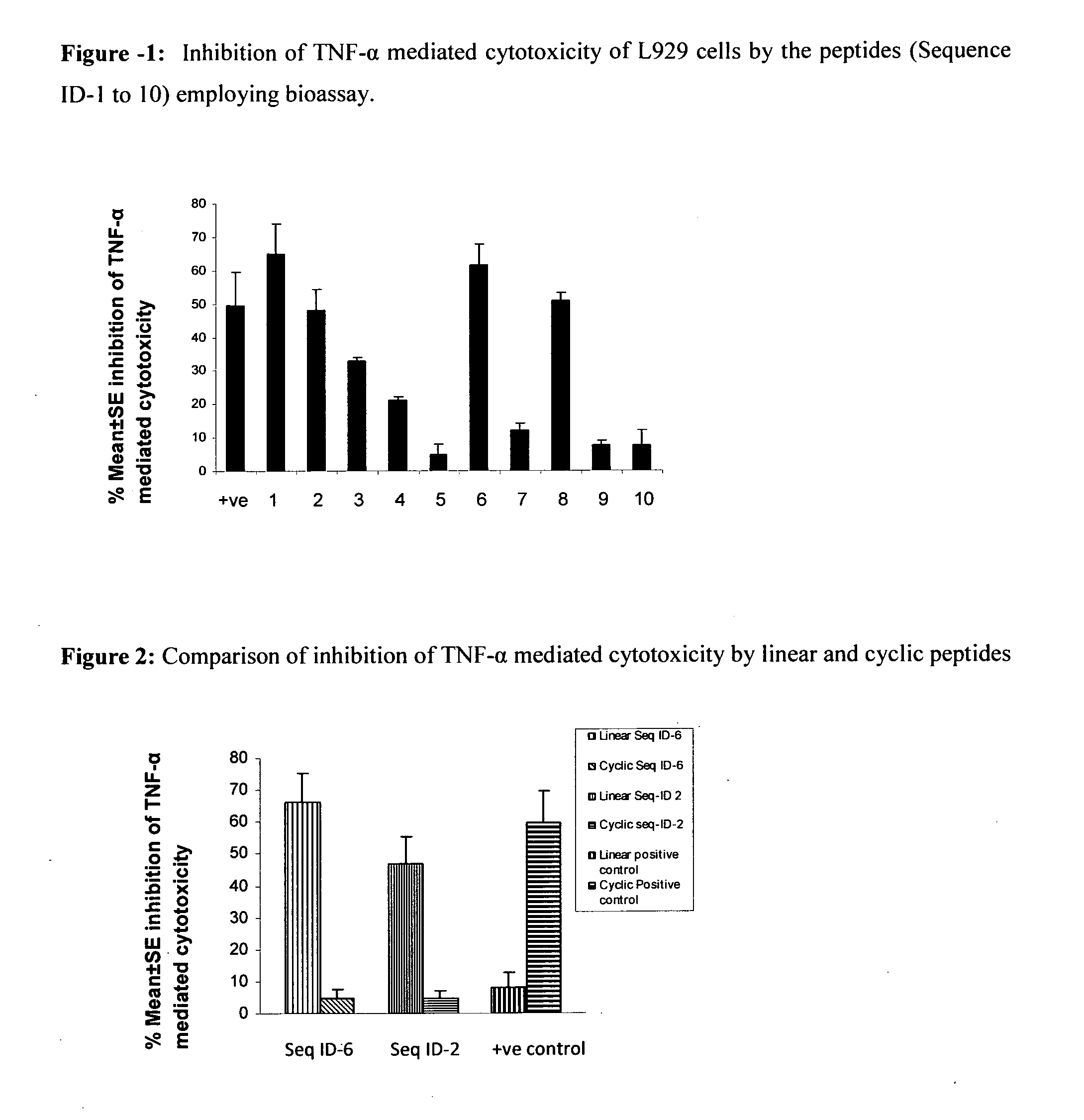
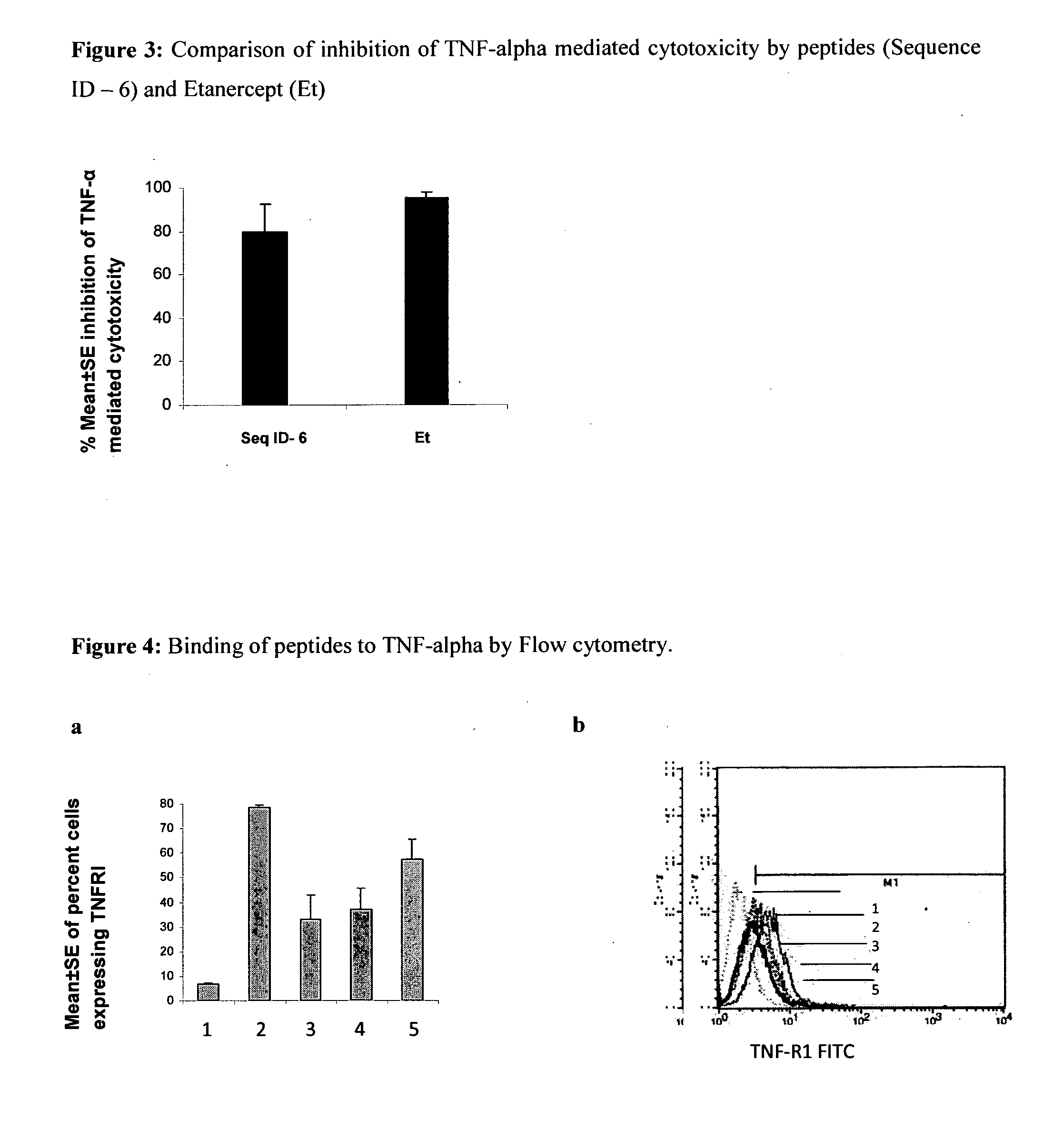
Tumor necrosis factor inhibiting peptides and uses thereof
The present invention relates to Tumor Necrosis Factor-alpha (TNF-alpha or TNF-α) inhibiting peptides and process for the preparation thereof. The present invention further relates to a pharmaceutical composition comprising TNF-alpha inhibiting peptides of the present invention and uses thereof in treating TNF-alpha mediated inflammatory disorders.
Owner:PANACEA BIOTEC
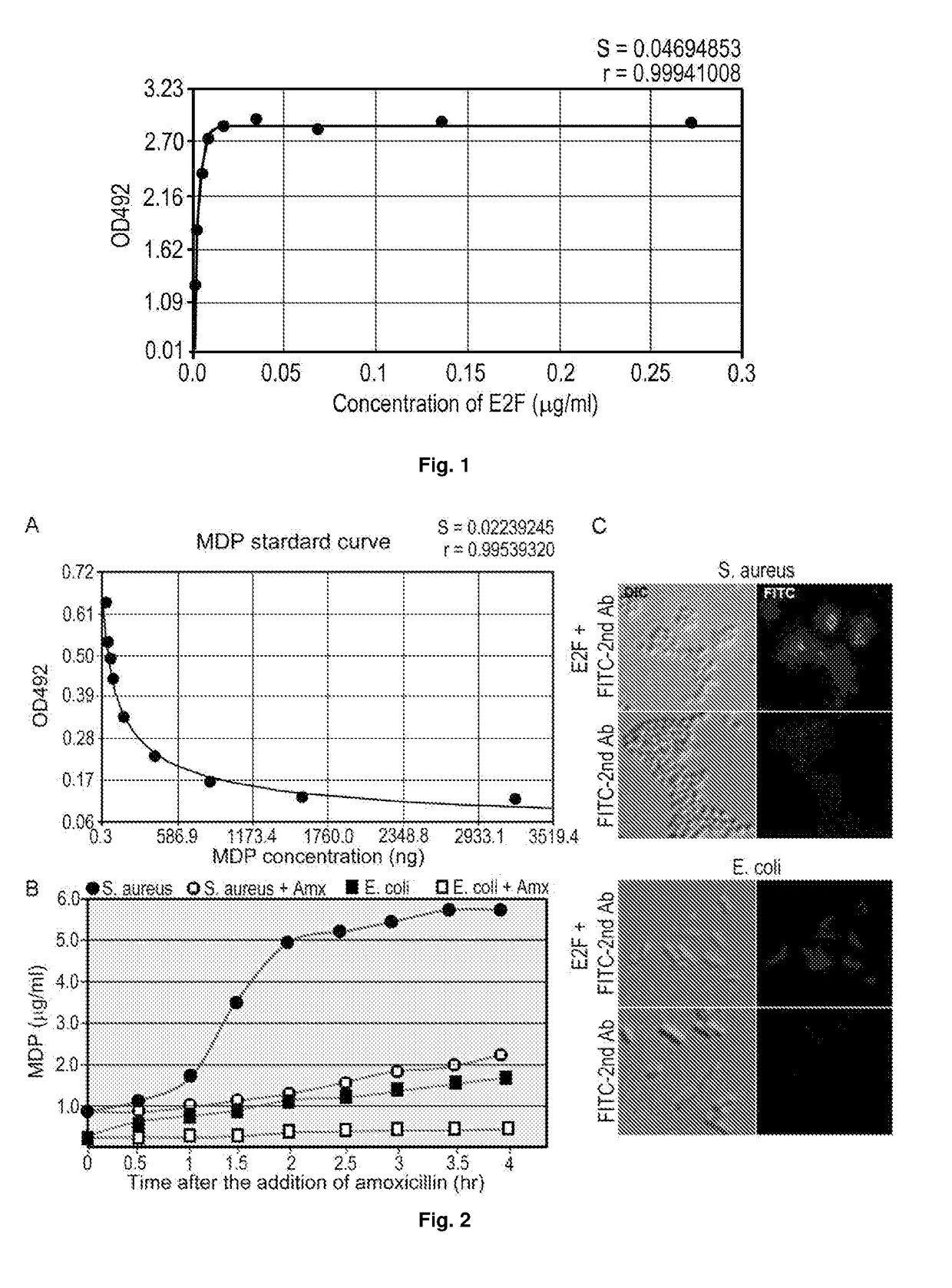
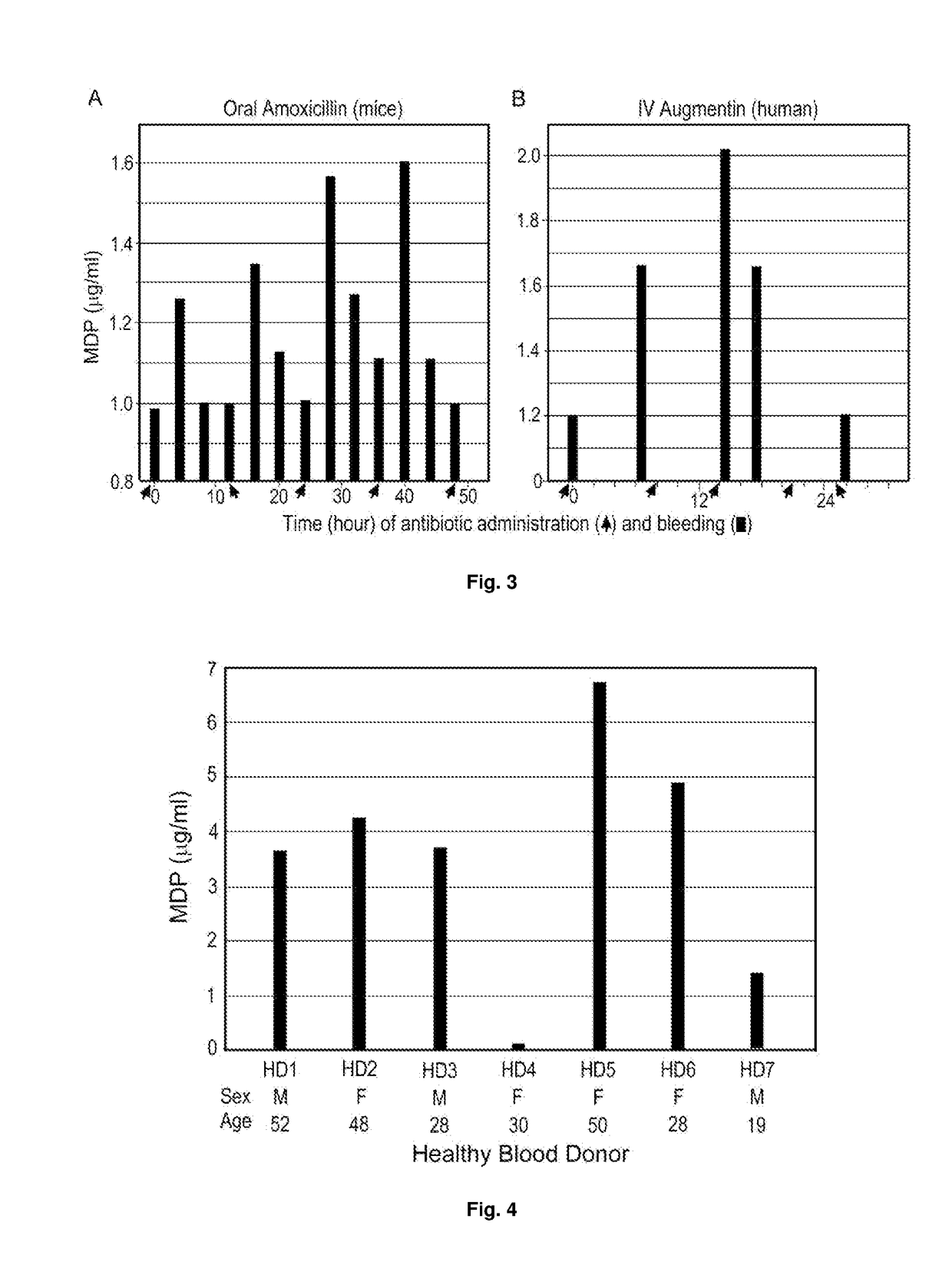
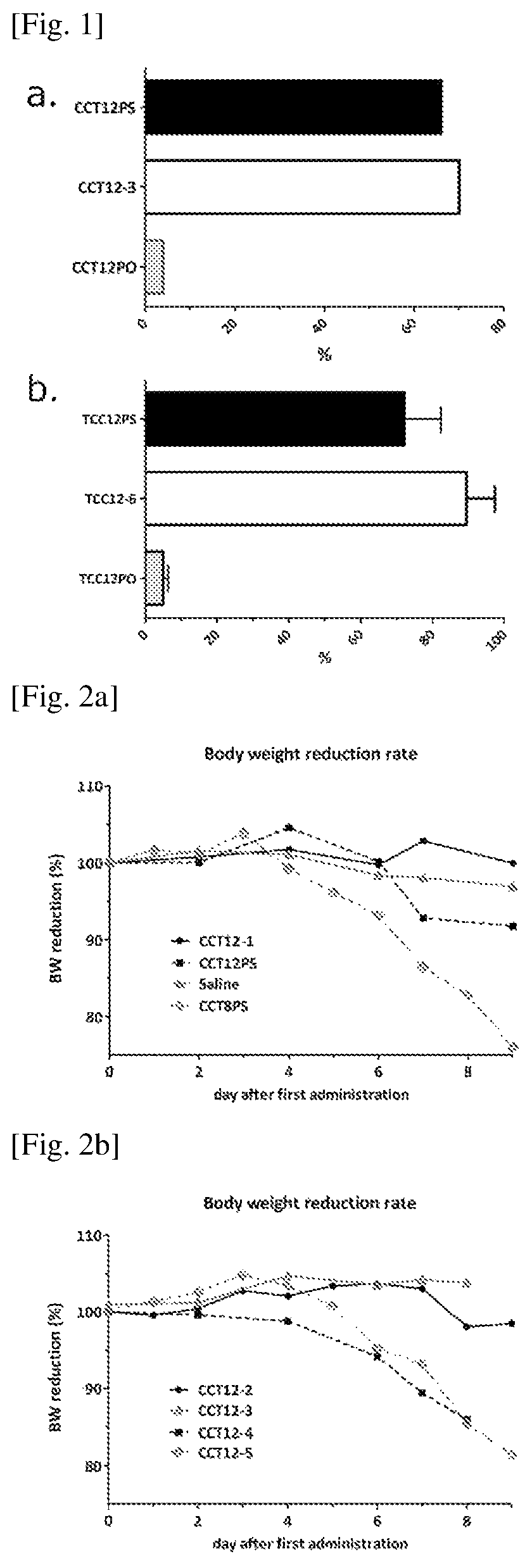
Monoclonal antibody against muramyl peptides in prevention and treatment of immune-mediated diseases
Disclosed are methods of treating an autoimmune or inflammatory disease using a composition comprising an isolated antibody or an antigen-binding fragment or variant thereof that is capable of binding to muramyl peptide, derivative, analog or salt thereof, wherein said muramyl peptide comprises muramic acid and an amino acid selected from the group consisting of alanine, isoglutamine, glutamic acid and a salt thereof. In one preferred embodiment, the composition can comprise of the isolated antibody or an antigen-binding fragment and one or more therapeutic agents such as Tumor Necrosis Factor (TNF) inhibitor. The autoimmune or inflammatory disease is selected from a group consisting of sepsis, septic shock, Crohn's disease, rheumatoid arthritis, asthma, allergy, atopic disorders, multiple sclerosis, pertussis, gonorrhea, inflammatory bowel disease, and antibiotic associated disorder.
Owner:AGENCY FOR SCI TECH & RES
Compositions and methods for ex vivo preservation of blood vessels for vascular grafts using inhibitors of tumor necrosis factor-alpha
InactiveUS20050201989A1Improve survivabilityAvoid damageBiocideDead animal preservationBlood levelTumor necrosis factor alpha
The present invention relates to ex vivo methods for preserving / maintaining blood vessels that are to be used as vascular grafts by inhibiting TNF-α. The present invention also relates to compositions comprising an inhibitor of a TNF-α for use in the methods of the invention, which compositions are free of active TGF-β or does not contain active TGF-β in an amount greater than endogenous blood levels.
Owner:ST CAMILLUS MEDICAL
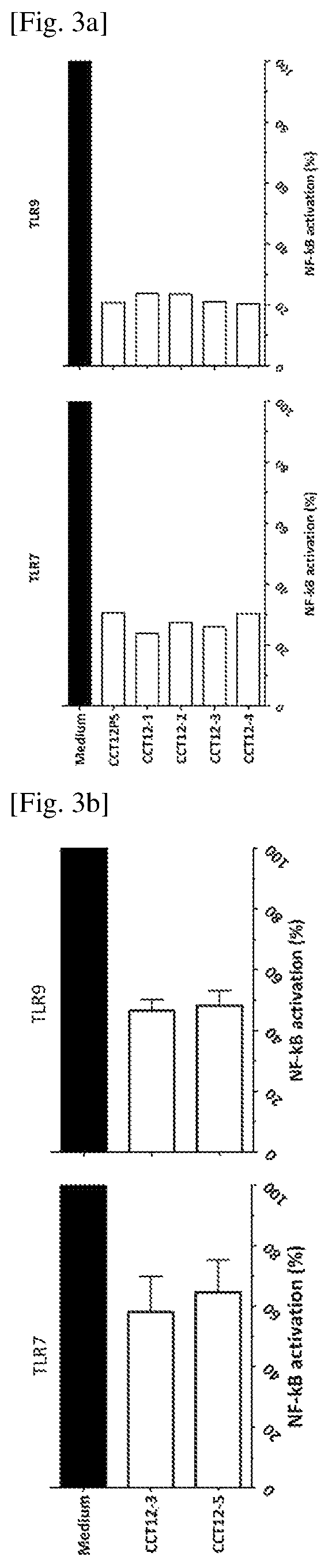
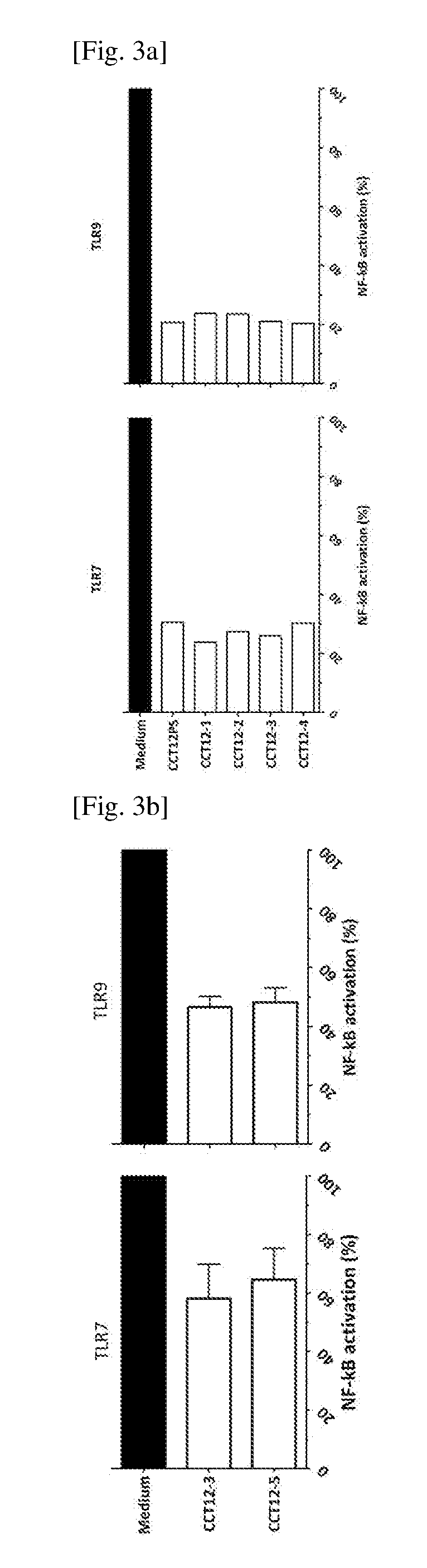
TLR inhibitory oligonucleotides and their use
ActiveUS10806749B2Increase doseSafer compositionOrganic active ingredientsActivity regulationDiseaseWhite blood cell
Owner:SBI BIOTECH CO LTD
Cosmetic composition
InactiveCN109833266APrevent agingDelay and improve aging problemsCosmetic preparationsToilet preparationsInflammatory factorsTraditional medicine
The embodiment of the invention discloses a cosmetic composition. The cosmetic composition comprises a plant extract having tumor necrosis factor inhibitor activity, a plant extract having antioxidative activity, and polypeptide having wrinkle resisting activity, wherein the polypeptide is selected from one or more of KTTKS, KTTKSF, GEKG, GEKGF, EEMQRR and EEMQRRF. Through mutual cooperation of various components, the cosmetic composition can start three ways of neutralizing inflammatory factors, eliminating oxygen free radicals and stimulating collagen tissue which is destructed or broken, intercellular substances and the like in a fibroblast proliferation derma layer after being used, so that the problem of skin ageing can be comprehensively prevented, delayed and alleviated, and the cosmetic composition has favorable technical effects and favorable application prospects.
Owner:深圳市富可迪生物科技有限公司 +1
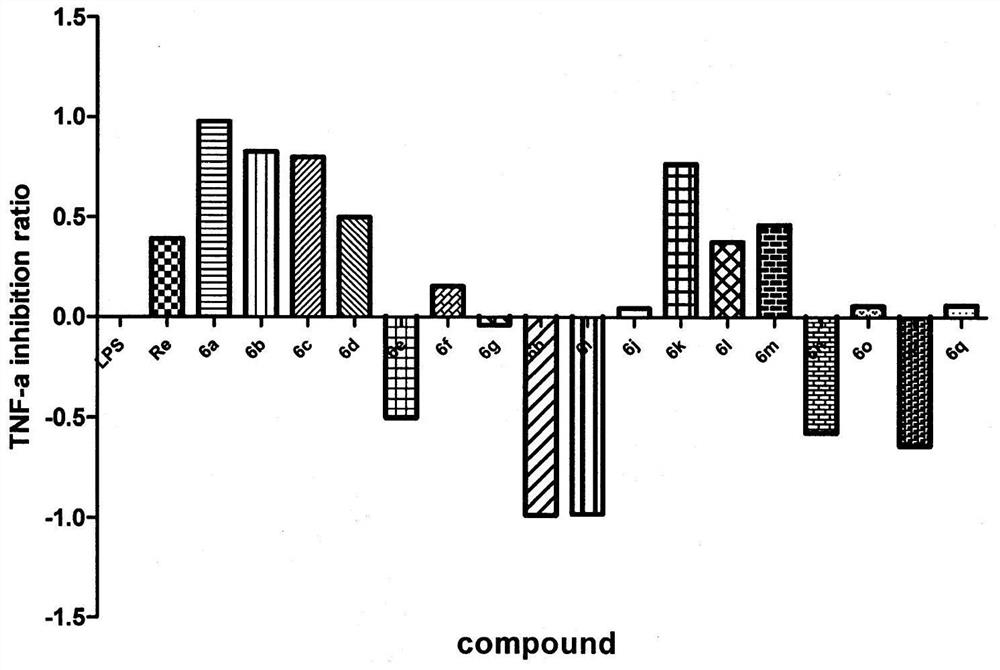
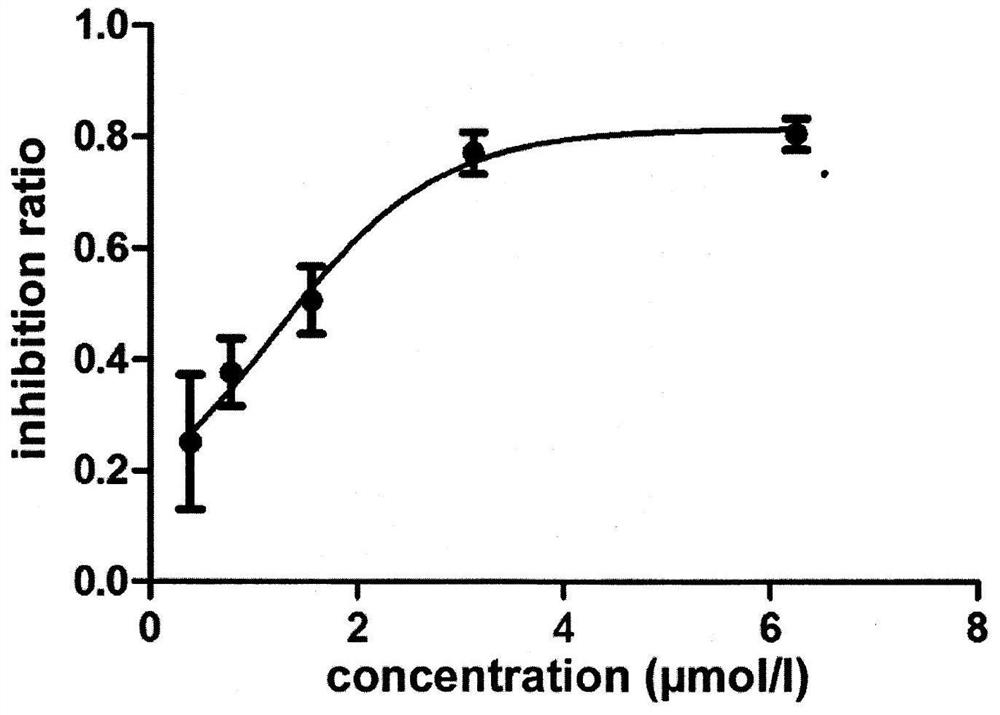
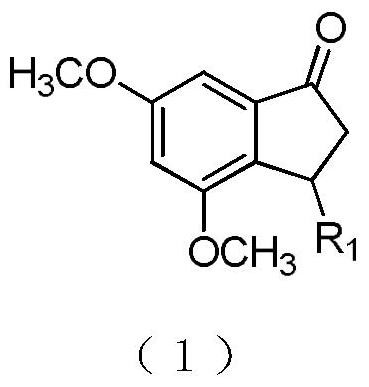
3-substituted-1-indanone derivative compound and its preparation method and pharmaceutical use
The invention belongs to the technical field of pharmaceutical chemistry and medicine, relates to a novel tumor necrosis factor (TNF-alpha) inhibitor and particularly relates to a small molecule 3-aryl-1-indanone derivative TNF-alpha inhibitor and its medicinal use. The invention provides the 3-substituted-1-indanone derivative compound shown in the following formula (1). An in-vitro experiment result shows that the prepared derivative compound has specific inhibition effects on a tumor necrosis factor (TNF-alpha) produced by LPS-stimulated macrophages RAW 264.7. The derivative can be used for preparation of a small molecule treatment drug for treating ulcerative colitis and as a small molecule TNF-alpha inhibitor and can be used for treating human TNF-alpha increasing-mediated immunoinflammatory diseases such as ulcerative colitis, Crohn's disease, rheumatoid arthritis, osteoarthritis and psoriasis.
Owner:FUDAN UNIV
Immunomodulation with novel pharmaceutical compositions
InactiveUS20060167054A1Lower Level RequirementsInhibition productionAntibacterial agentsBiocideHydrogenImmunomodulations
Owner:MEDIQUEST THERAPEUTICS INC
Tlr inhibitory oligonucleotides and their use
ActiveUS20190008888A1Increase doseSafer compositionOrganic active ingredientsActivity regulationMultiple organ dysfunction syndromeDisease
The inhibitory oligonucleotides with partial phosphorothioation with reduced toxicity strongly block NF-kB activation induced by TLR9 agonists and TLR7 / 8 agonists. The production of proinflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFa), is inhibited by the inhibitory-oligonucleotides. Interferon (IFN) production from human PBMC induced by TLR9 agonist is prevented by the inhibitory-oligonucleotides. These oligonucleotides can be used as a remedy for the treatment of immune-mediated disorders such as rheumatoid arthritis, systemic lupus erythematosus (SLE), sepsis, multiple organ dysfunction syndromes and inflammatory cytokine-mediated inflammatory disease.
Owner:SBI BIOTECH CO LTD
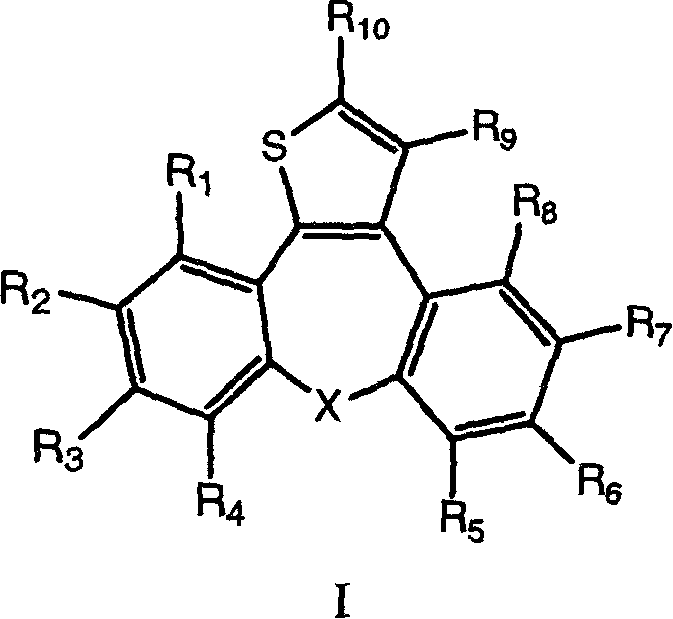
Thienodibenzoazulene compounds as tumor necrosis factor inhibitors
The present invention relates to the dibenzoazulene compounds represented by formula I as well as to their pharmaceutical preparations for the inhibition of tumor necrosis factor alpha (TNF-alpha) and interleukine 1 (IL-1) in mammals at all diseases and conditions where these mediators are excessively secreted. The compounds of the present invention also demonstrate an analgetic action and can be used to relieve pain.
Owner:PLIVA ISTRAZIVACKI INST D O O
Methods and compositions for the treatment of an inflammatory bowel disease
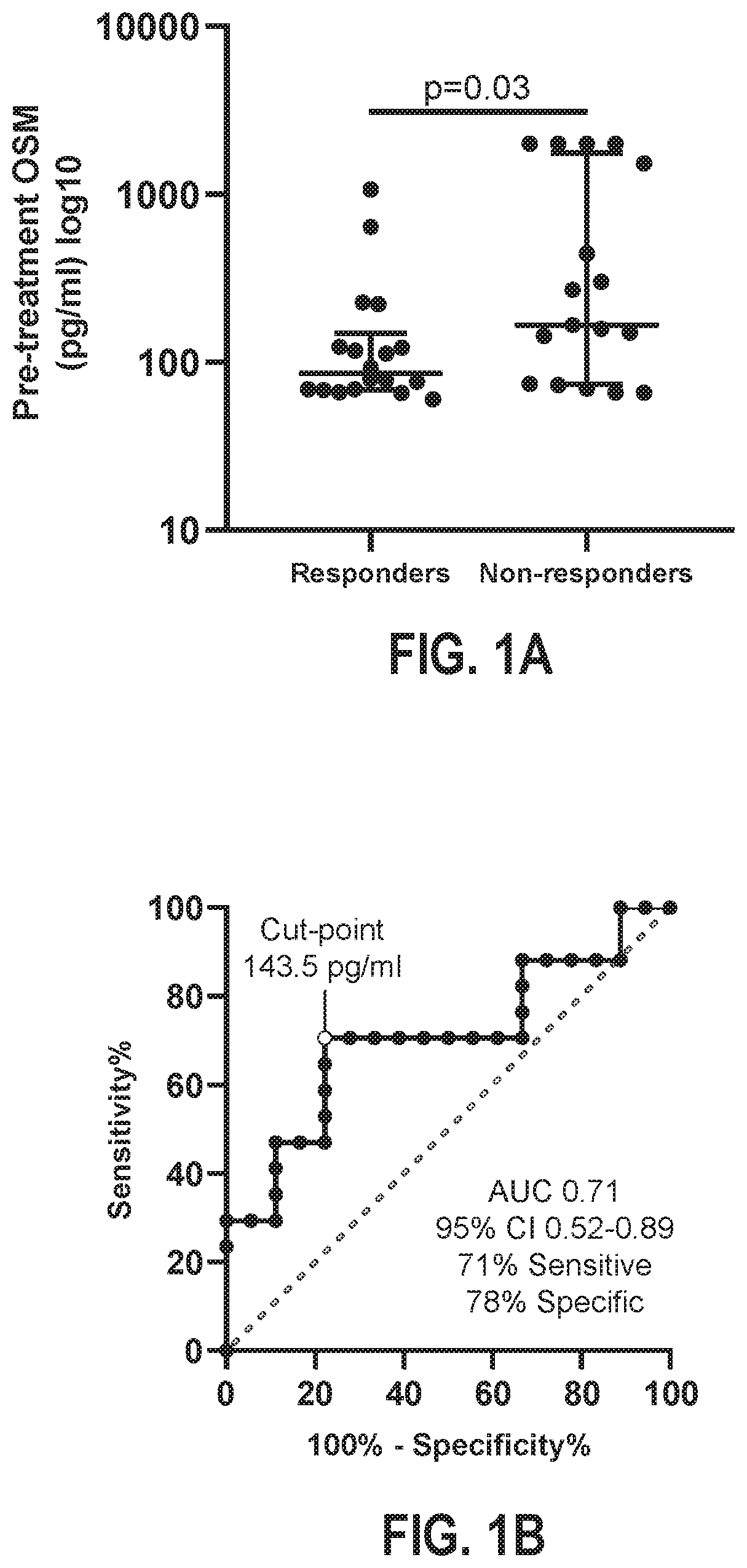
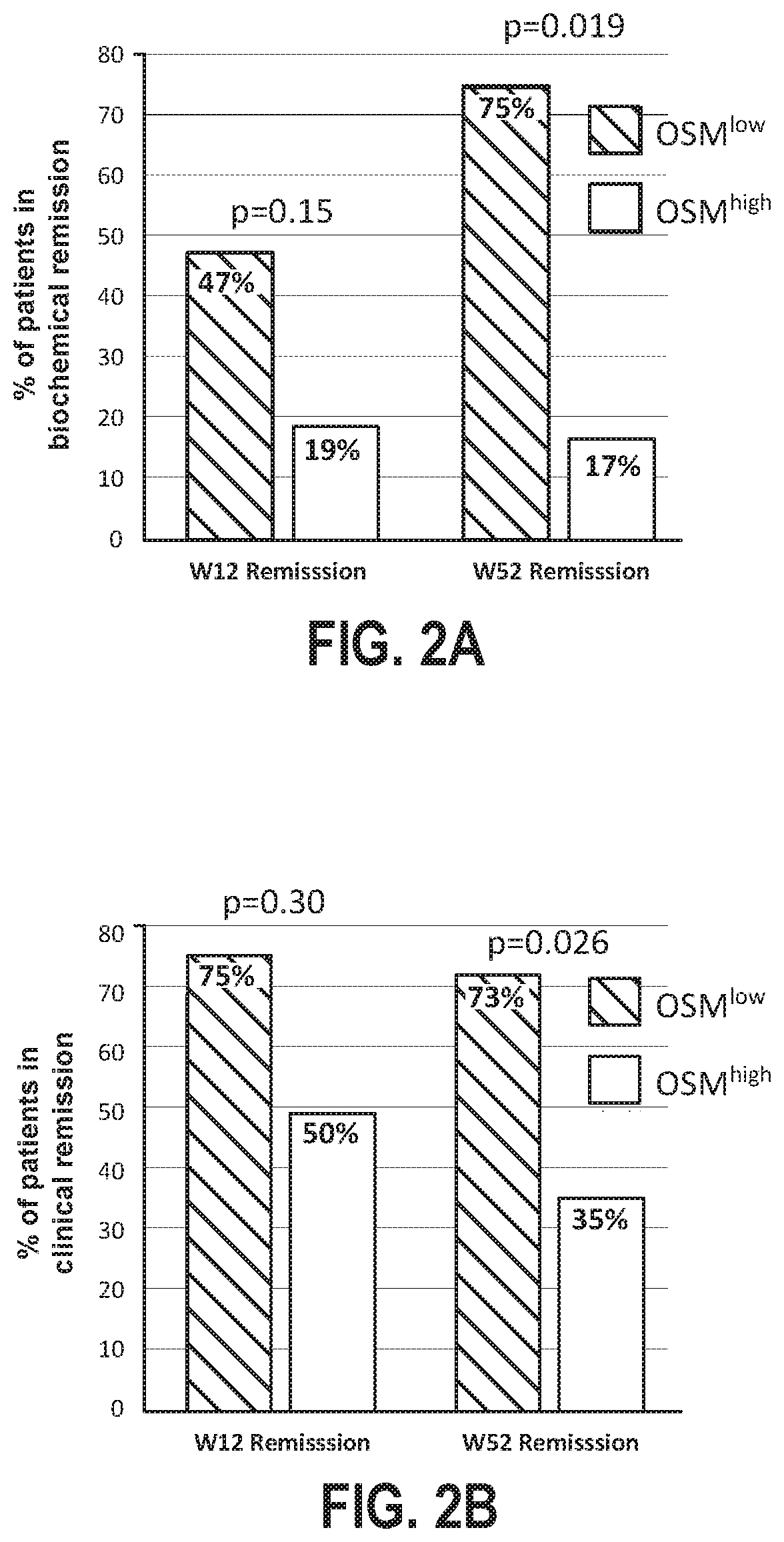
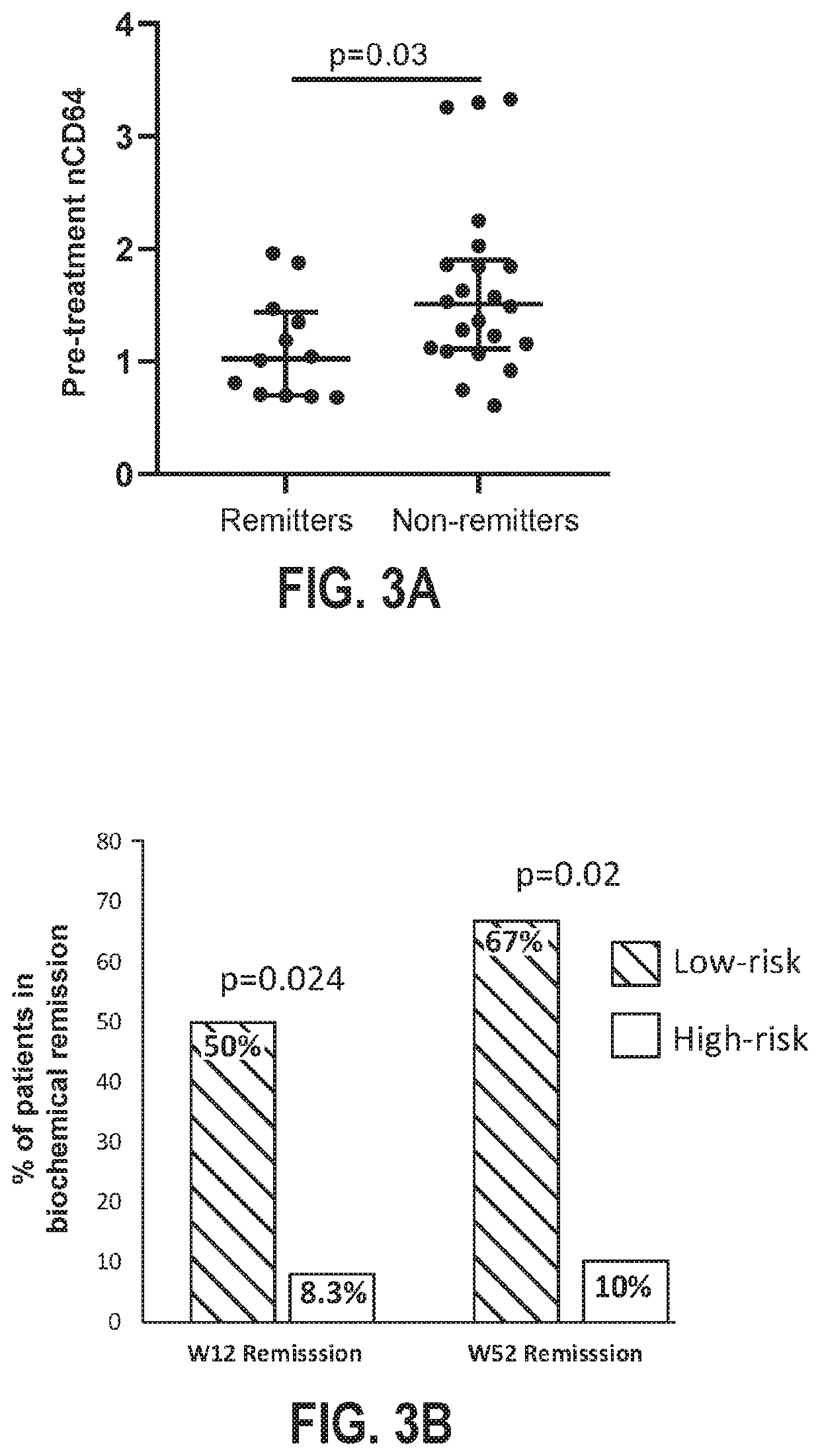
Disclosed herein are methods for the treatment of an individual having an inflammatory bowel disease (“IBD”). The disclosed methods may include the detection of Oncostatin M (OSM) in a biological sample obtained from an individual having an IBD. The detection of OSM may be used to characterize the individual as a tumor necrosis factor (TNF) inhibitor (TNFi) responder or a TNFi non-responder for selection of appropriate treatment.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
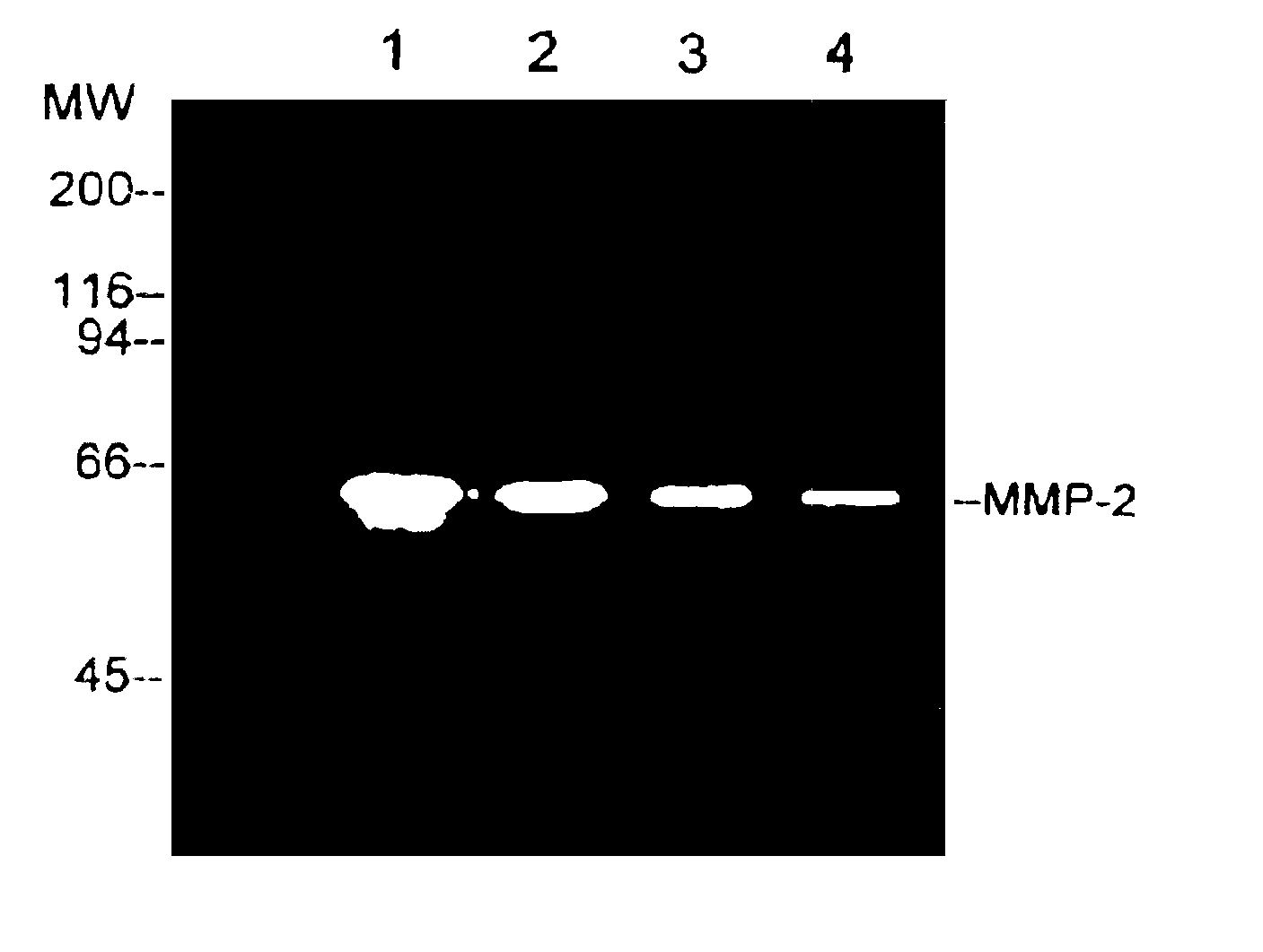
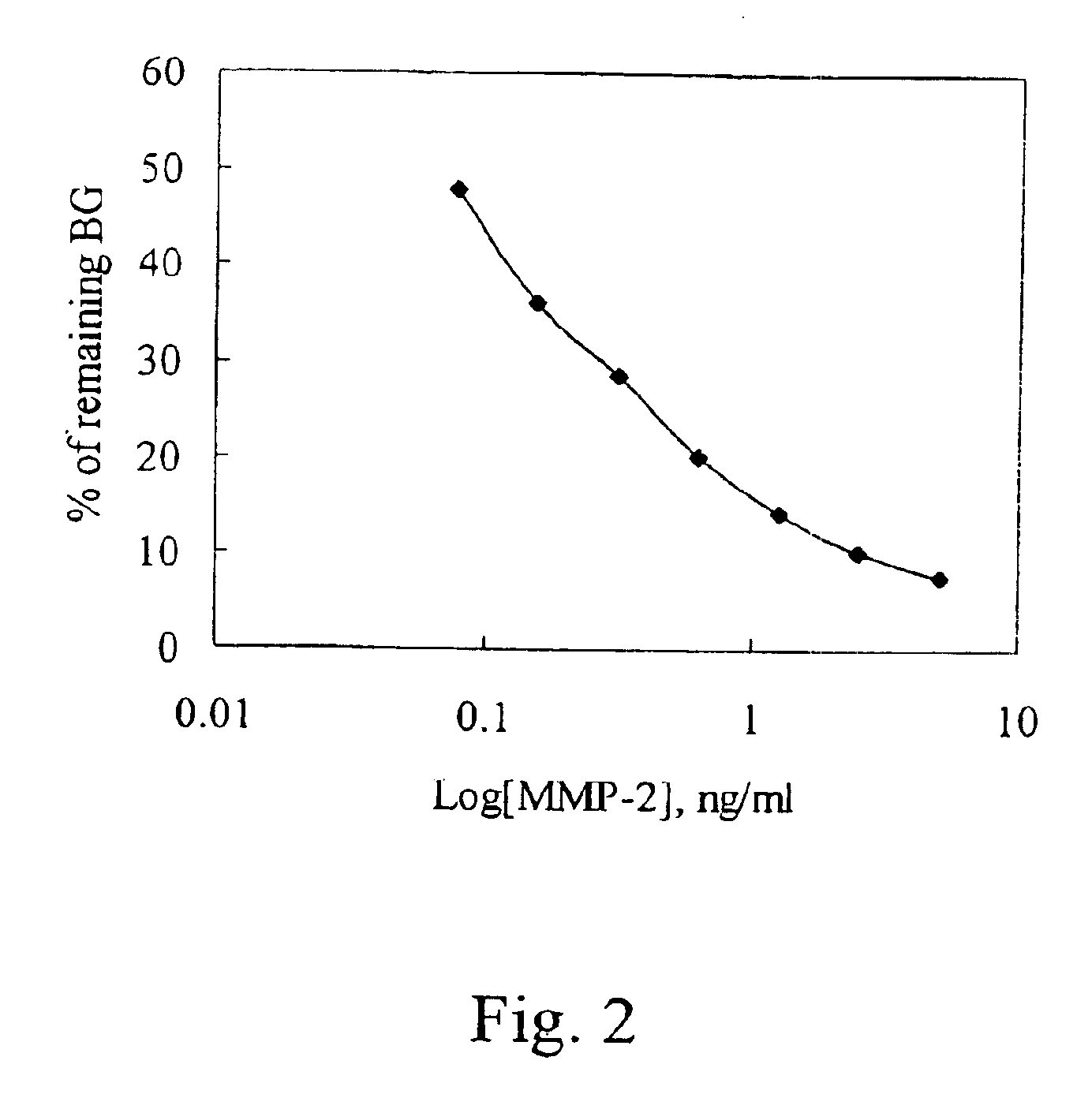
Matrix metalloproteinase and tumor necrosis factor inhibitors
A compound of formula 1-[3,4-dihydroxy-5-(2-hydroxyethyl)tetrahydrofuran-2-yl]pryimidine-2,4(1H,3H)-dione has inhibitory effects of matrix metalloproteinase-2 (gelatinase A) enzyme and binding of TNFα to TNFα-RI.
Owner:YU CHEN
Methods For Determining Anti-TNF Therapeutic Response
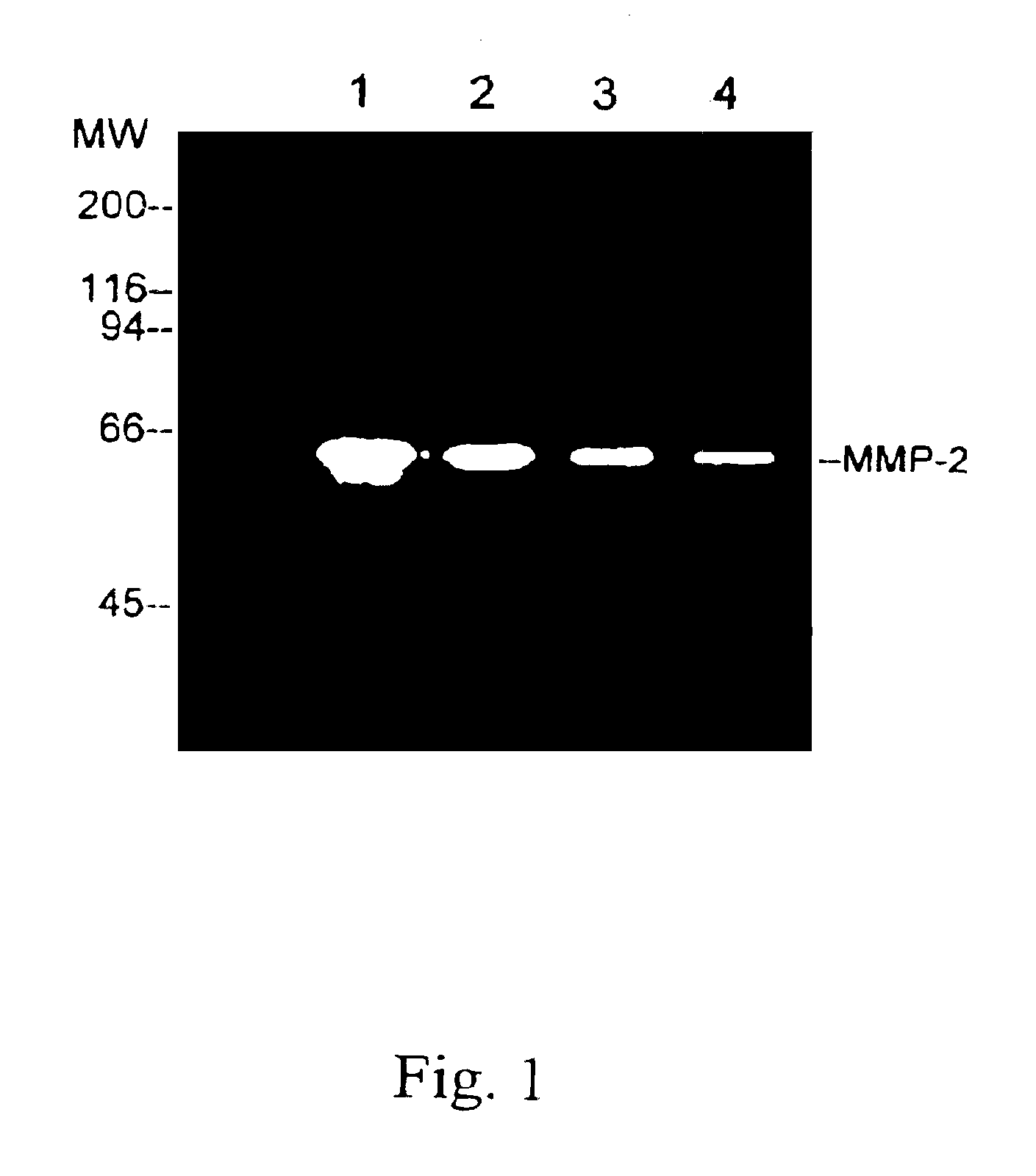
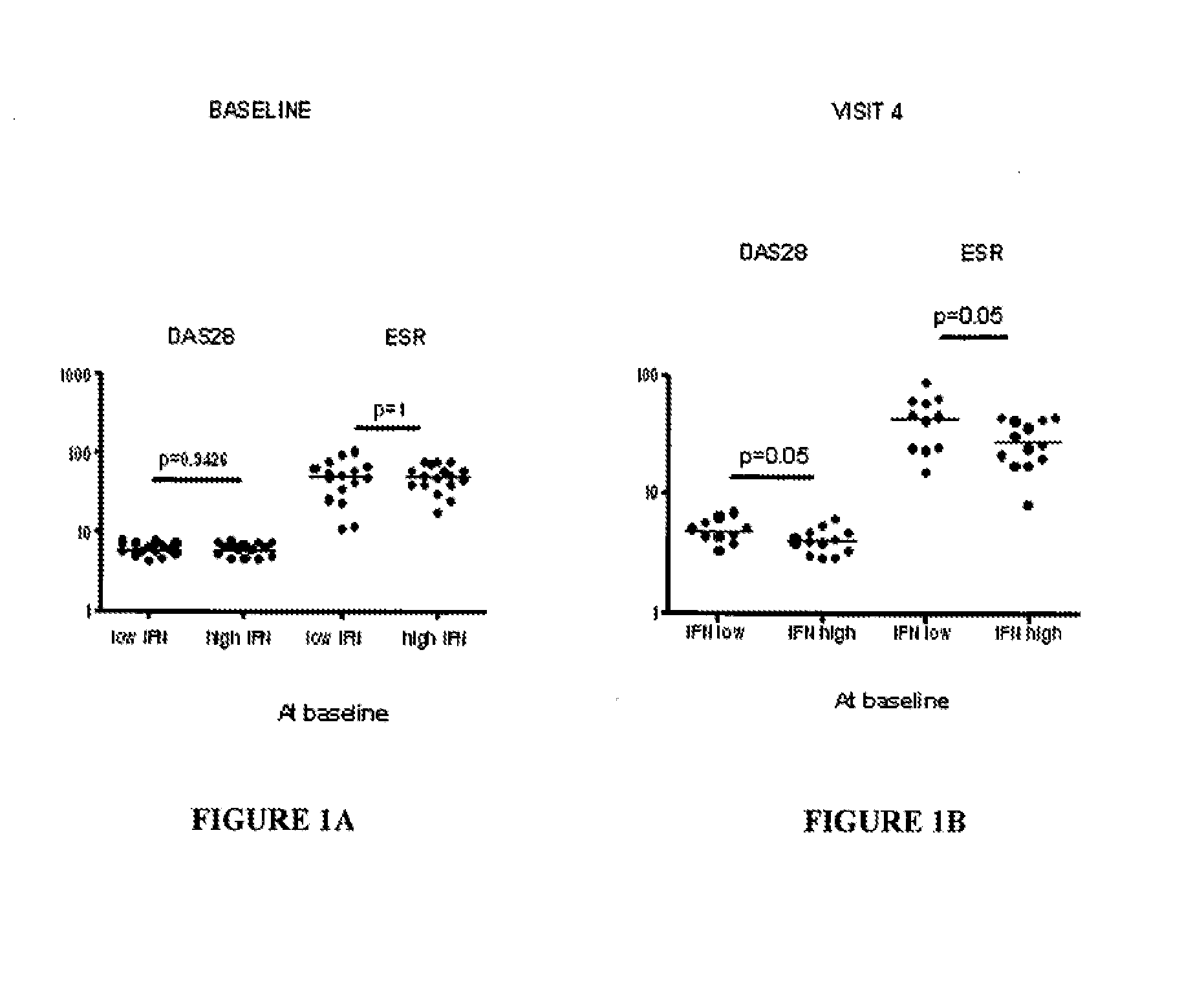
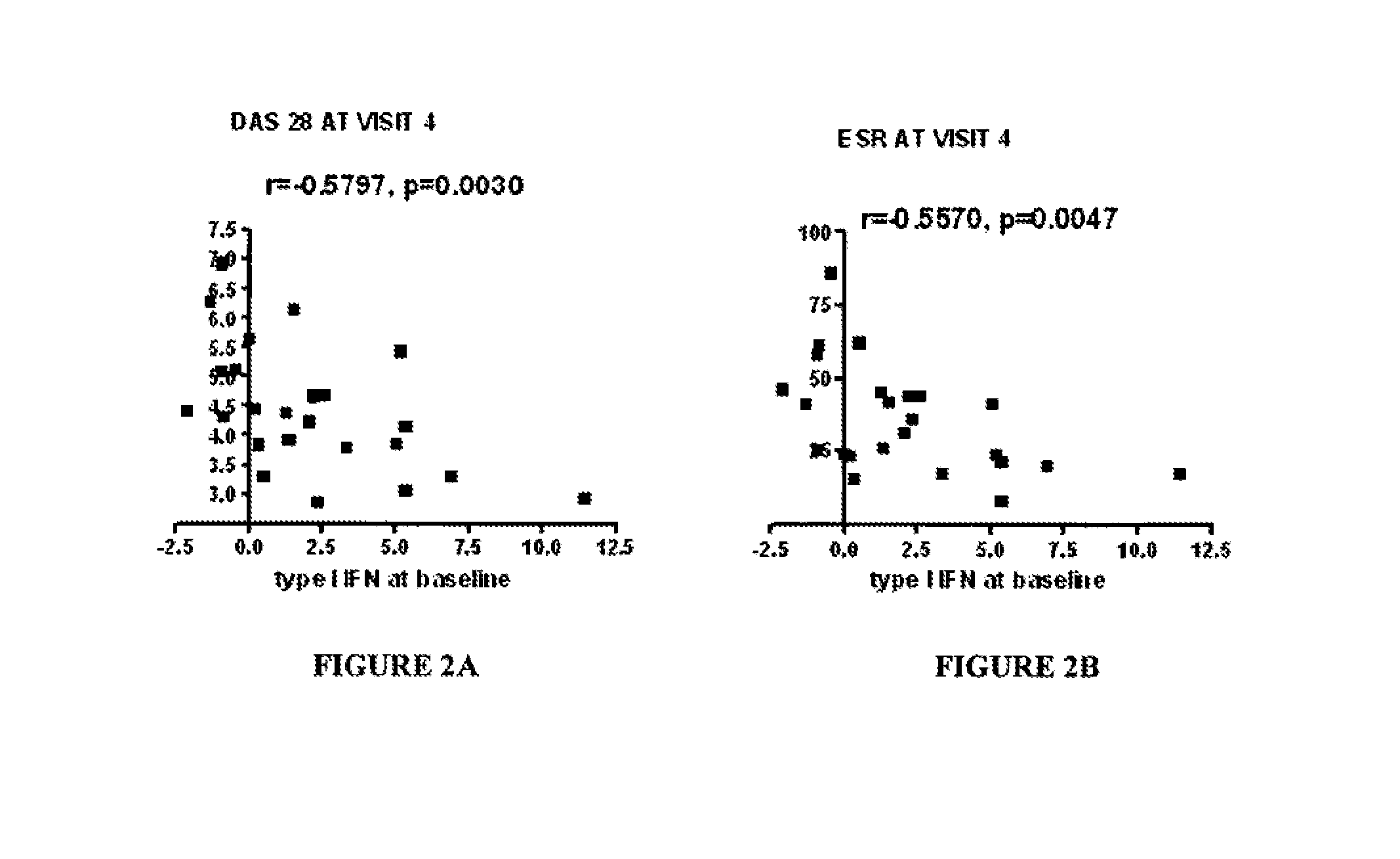
InactiveUS20150160236A1Microbiological testing/measurementDisease diagnosisAnkylosing spondylitisSystemic lupus erythematosus
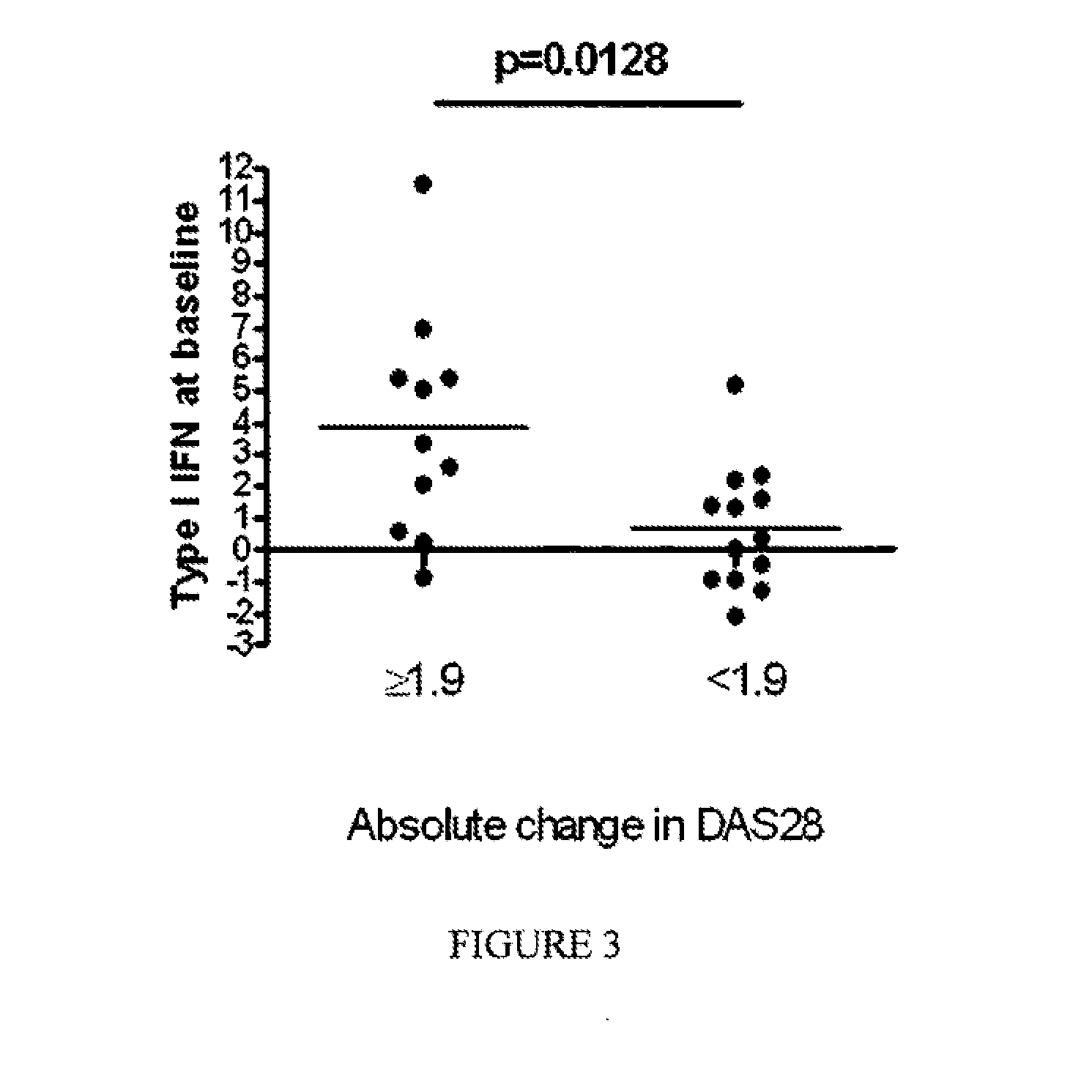
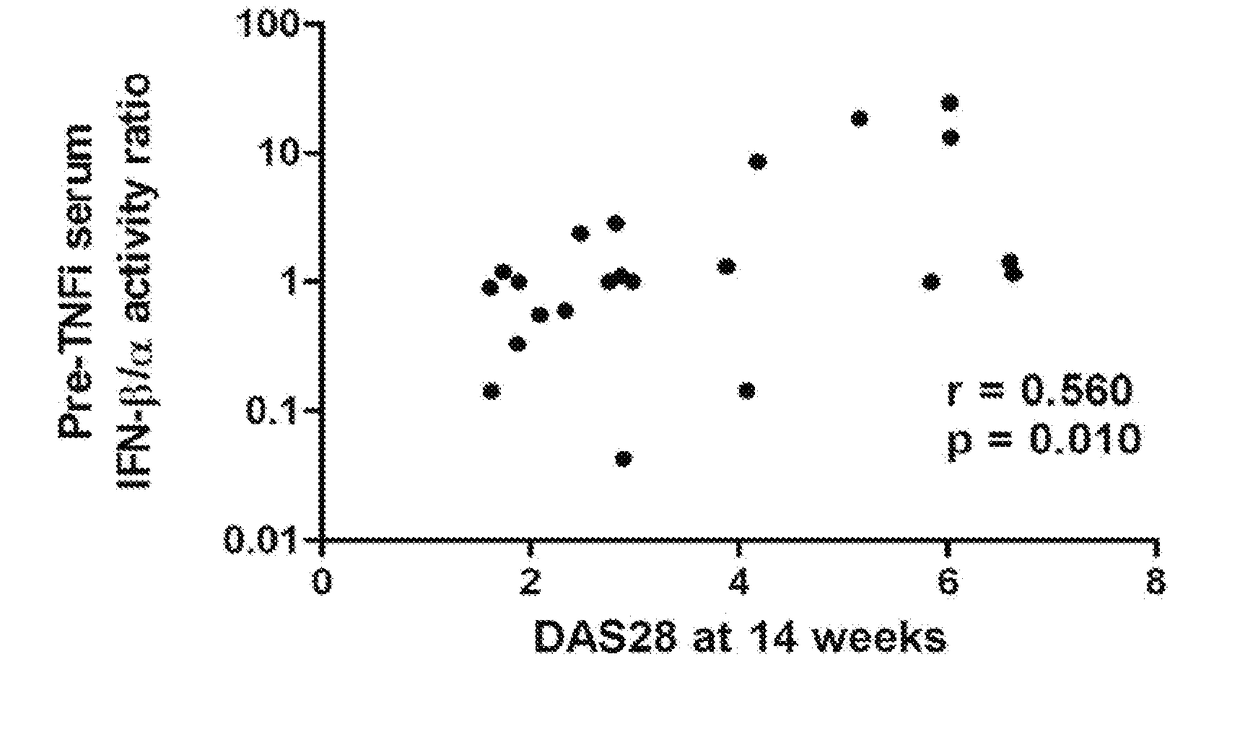
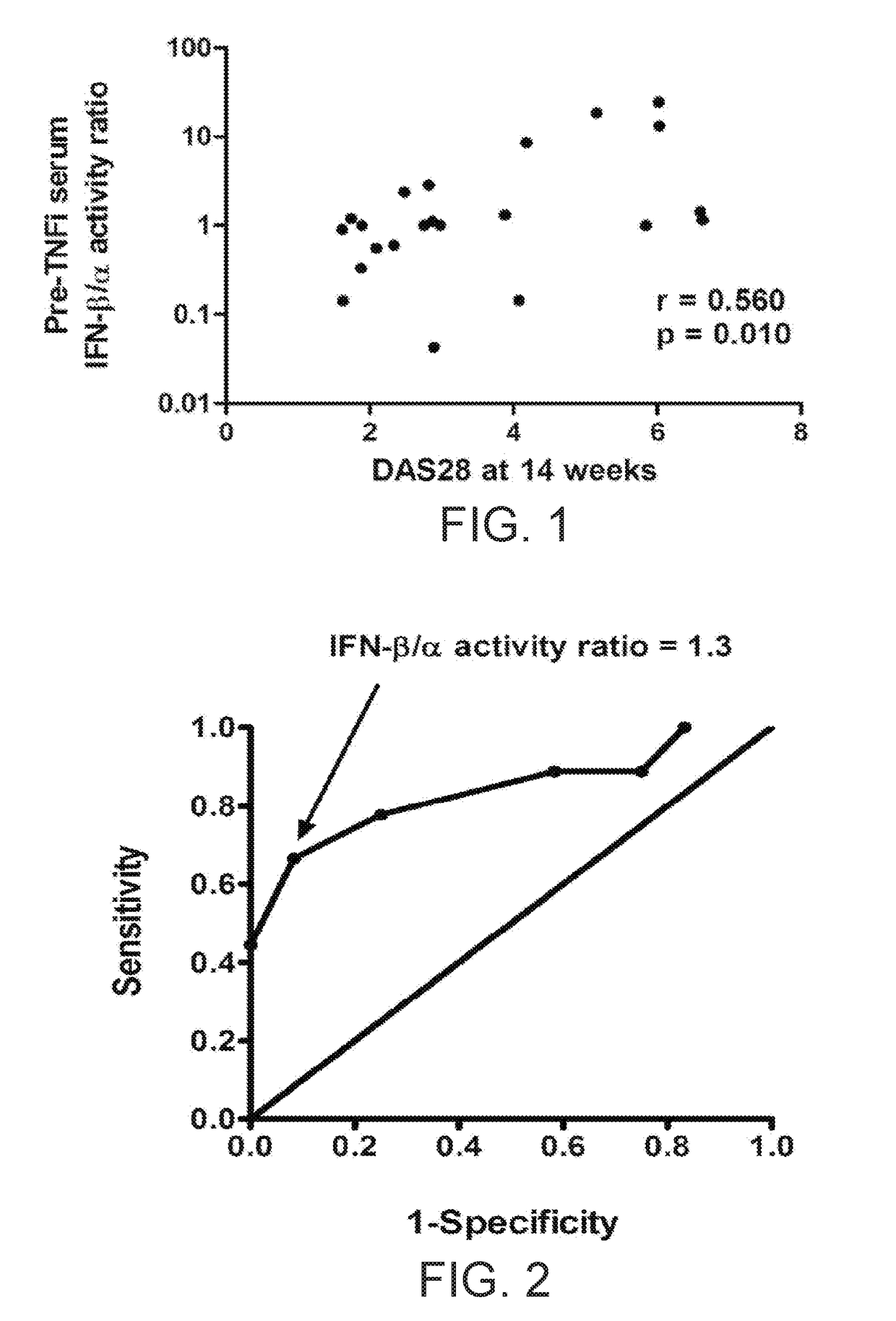
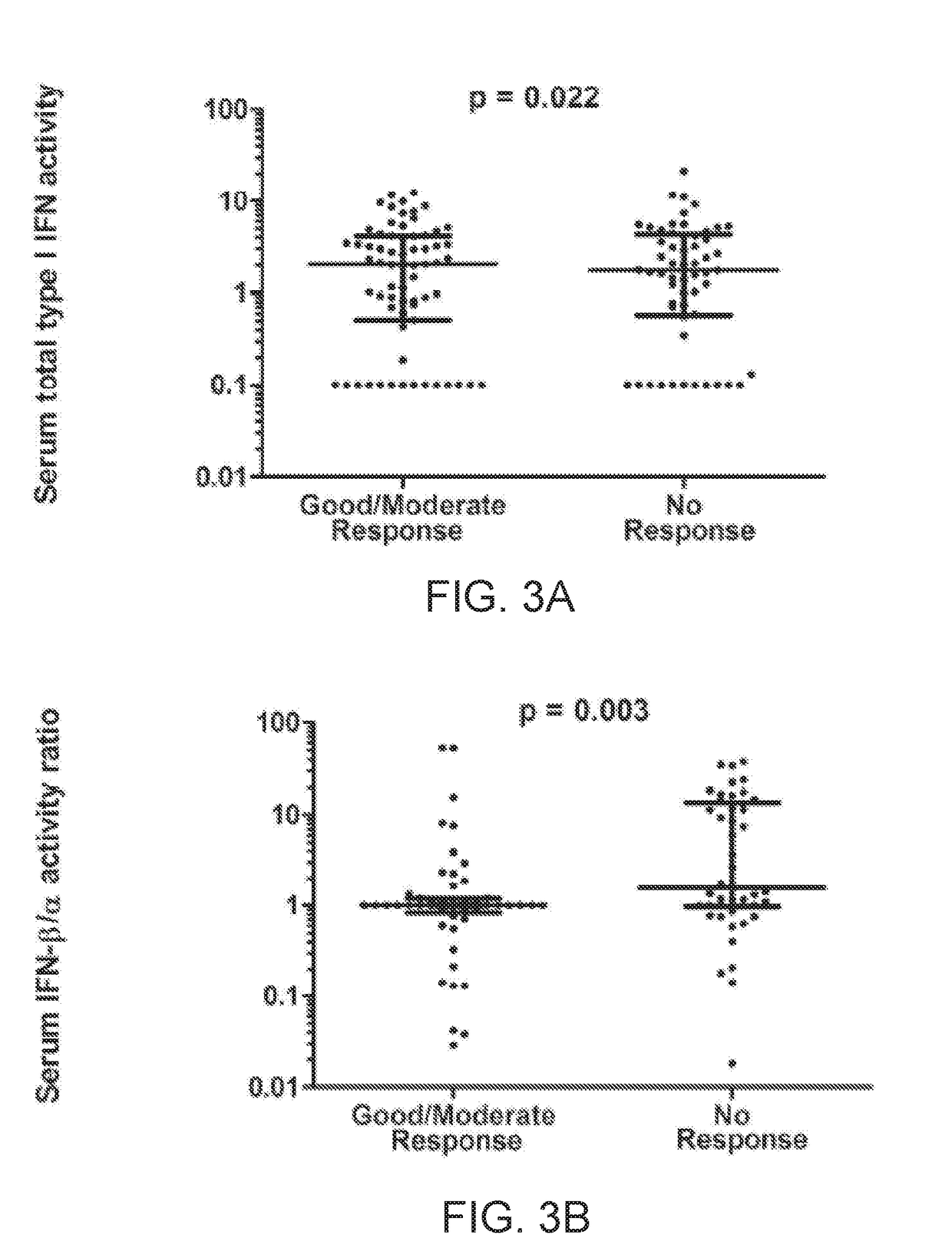
The present invention relates to methods for identifying patients that will respond to treatment with anti-TNF-therapy, i.e., anti-TNF responder patients. In particular, the present invention relates to determining response to an inhibitor of tumor necrosis factor (TNF) in a patient with a chronic inflammatory disease by determining the activity of type I interferon in the patient. The invention further relates to quantification of type I interferon as a measure of predicting responsiveness to anti-TNF therapy in patients with a chronic inflammatory disease such as rheumatoid arthritis (RA), psoriatic arthritis, ankylosing spondylitis, juvenile chronic arthritis, lupus, Crohn's disease, as well as for cardiovascular disease.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY
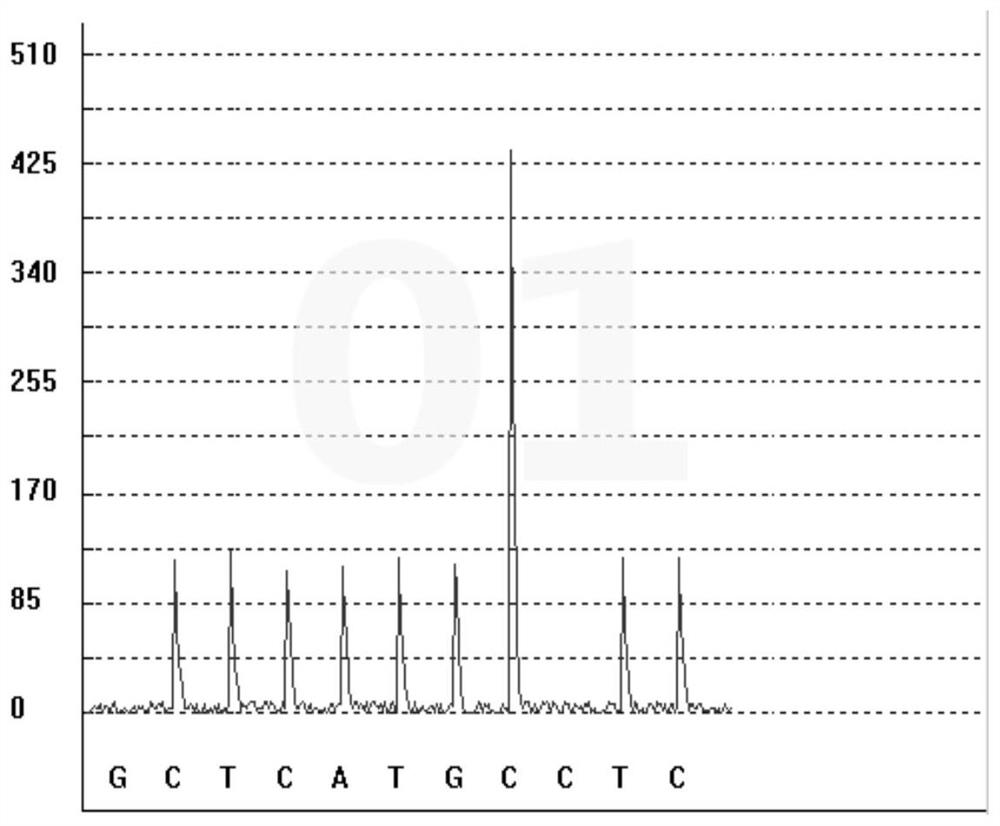
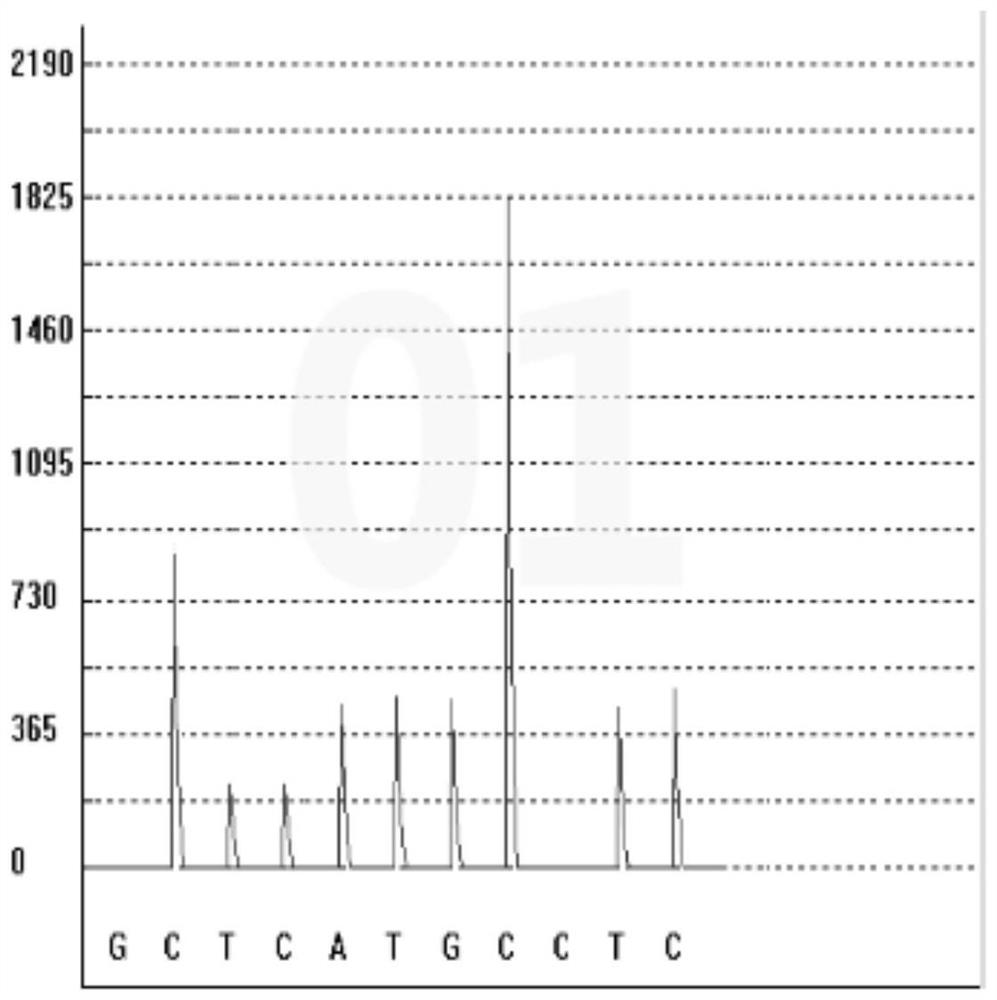
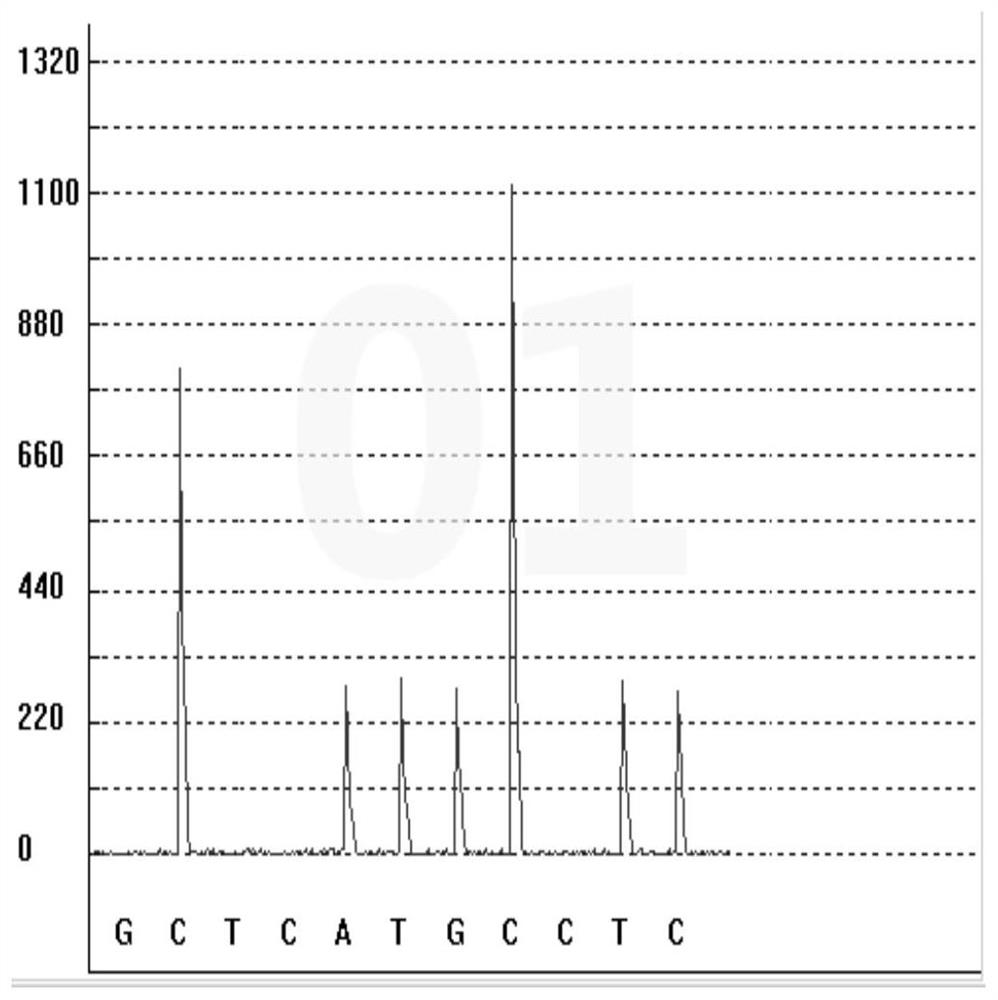
Gene polymorphism detection kit for predicting efficacy of tumor necrosis factor alpha inhibitor as well as detection method and application of gene polymorphism detection kit
InactiveCN113667745ASimplify the sequencing workflowSimplify timeMicrobiological testing/measurementDNA/RNA fragmentationTumor necrosis factor alphaEfficacy
The invention discloses a gene polymorphism detection kit for predicting the efficacy of a tumor necrosis factor alpha inhibitor as well as a detection method and application of the gene polymorphism detection kit. The efficacy of the tumor necrosis factor alpha inhibitor reflects the metabolic capability by detecting the polymorphism of a TNF alpha G308A gene; according to the kit, a specific amplification primer and a sequencing primer are designed aiming at the polymorphism of TNF alpha G308A, and the kit comprises the following components: a sample treatment solution, magnetic beads, an amplification reagent 1, an amplification reagent 2, the TNF alpha G308A sequencing primer and a positive control. On one hand, a mode of extracting and amplifying in the same tube is adopted, so that the risk of nucleic acid loss due to repeated tube transfer during extraction is avoided; on the other hand, rapid isothermal amplification is carried out by adding an anti-inhibitor; the invention aims to detect the gene polymorphism of the efficacy of the tumor necrosis factor alpha inhibitor by combining constant-temperature PCR (Polymerase Chain Reaction) and pyrophosphoric acid detection, and provides suggestions from the gene perspective for clinical personalized medication.
Owner:上海普然生物科技有限公司
Tumor necrosis factor inhibitory polypeptide and application thereof
InactiveCN105111284AInhibition of physiologyInhibition of pathological effectsPeptide/protein ingredientsAntipyreticMedicineDrug development
The invention relates to the field of drugs, particularly a polypeptide capable of inhibiting tumor necrosis factor and preventing and treating rheumatoid arthritis. The sequence is RINDISHTQSVSSKQKVTG and is a brand-new sequence. The polypeptide can be used for treating rheumatoid arthritis, and has potential new drug development value.
Owner:SUZHOU PULUODA BIOLOGICAL SCI & TECH
Reagents and methods for predicting efficacy of tumor necrosis factor inhibitors
ActiveCN107817353BEasy to answerComponent separationMaterial analysis by electric/magnetic meansSpondarthritisEfficacy
Owner:FUDAN UNIV
Methods and materials for treating autoimmune conditions
InactiveUS20180321235A1Organic active ingredientsMicrobiological testing/measurementWilms' tumorJanus kinase
This document provides methods and materials involved in treating autoimmune conditions. For example, methods and materials for using either (a) one or more tumor necrosis factor alpha (TNF-α) inhibitors or (b) one or more Janus kinase (JAK) inhibitors to treat autoimmune conditions such as rheumatoid arthritis are provided.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Application of substituted aryl hydrazone compound serving as anti-tumor necrosis factor inhibitor medicament
ActiveCN101766602BEffective pharmacological activityOrganic active ingredientsAntineoplastic agentsDepressantAnkylosing spondylitis
The invention relates to clinical application of a substituted aryl hydrazone compound and a derivative thereof serving as an anti-tumor necrosis factor inhibitor. A test result shows that the compound has a lower cytotoxicity in animal bodies, so the medical compound is expected to be used as an anti-TNF-alpha and TNF-beta inhibitor in the clinical application and is mainly used for treating some TNF related immunological diseases such as rheumatic arthritis, inflammatory bowel diseases, diabetes, hematosepsis, psoriasis, ankylosing spondylitis and some communicable diseases including HIV. Acurrent TNF related research also shows that the medical compound can be used for treating clinical blood and solid tumor such as myelodysplastic syndrome, myelofibrosis, acute bone marrow cell leucocythemia, acute / chronic graft-versus-host reaction, ovarian cancer, nephrocyte cancer and the like.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com