3-substituted-1-indanone derivative compound and its preparation method and pharmaceutical use
A compound, indanone technology, applied in the field of small molecule 3-aryl-1-indanone derivatives TNF-α inhibitors, new tumor necrosis factor inhibitors, can solve the problems of induction of immune response, high price, failure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
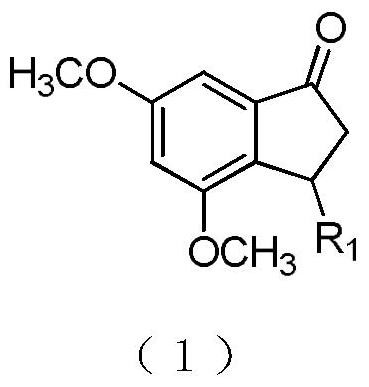
[0017] Example 1: Synthesis of 3-substituted-1-indanone derivatives
[0018]
[0019] Step 1: Take two-neck bottles, N 2 Protect, vacuumize, first add methyl p-methyl sulfone, stir and dissolve with anhydrous THF at -78°C, slowly add LDA (1.2eq) and react for 1h, add 1 (1.5eq), stir overnight, TLC detection (PE: EA=2:1) Whether the raw material has reacted completely; after the reaction is completed, concentrate under reduced pressure, extract with EA, and saturate NH 4 Cl, water, saturated NaCl solution washing, organic layer anhydrous NaSO 4 Dry, concentrate, and purify on a silica gel column (PE:EA=2:1—1:1) to obtain the target compound 2 in the form of white powder;
[0020] Step 2: Take two neck bottles, N 2 Protect, vacuumize, add 2 and aldehyde 3 (1.2eq), stir and dissolve with anhydrous toluene, add piperidine (0.2eq) and acetic acid (0.2eq), reflux and stir for 6h-8h, TLC detection (PE:EA=3 : 1) Whether the raw material has reacted completely; after the react...
Embodiment 2
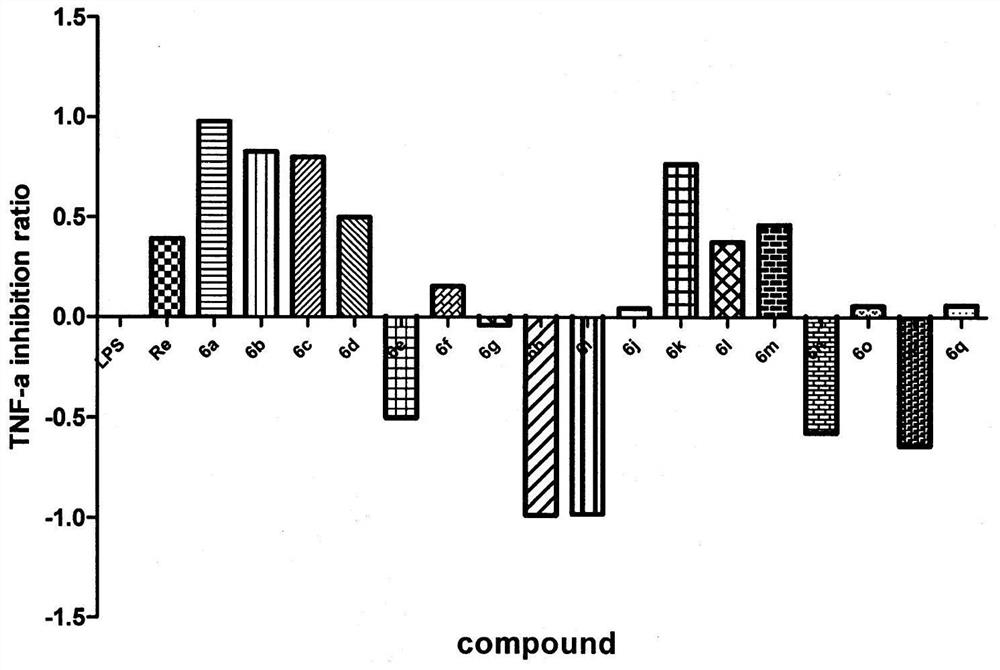
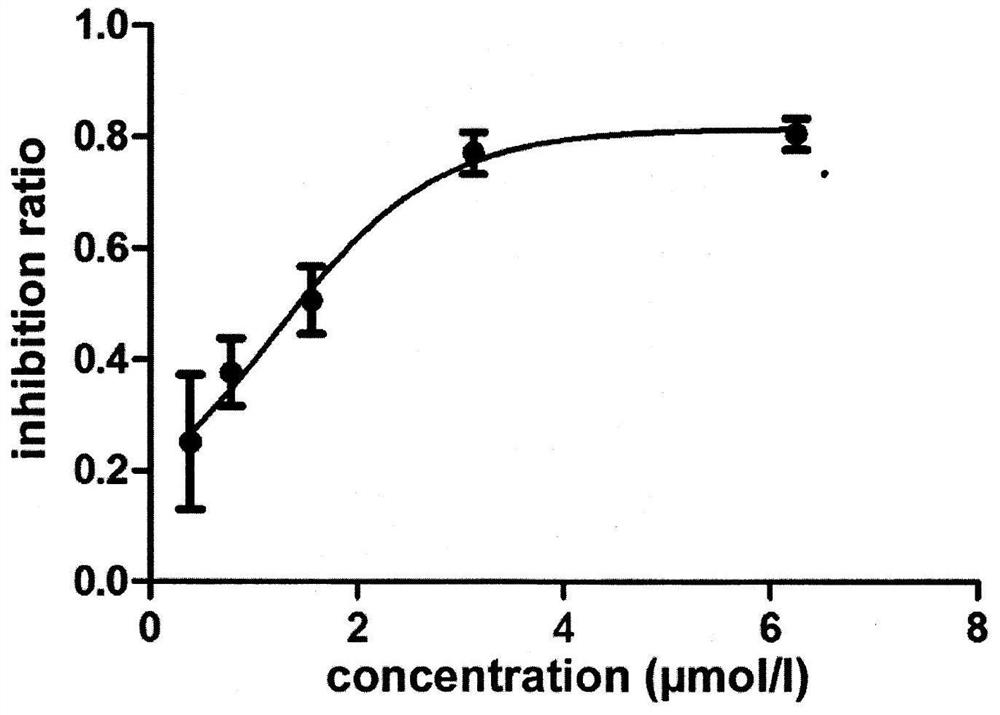
[0058] In vitro inhibitory activity of 3-substituted-1-indanone derivatives on tumor necrosis factor (TNF-α) released by LPS-stimulated macrophage RAW264.7:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com