Application of bacteroides fragilis to prevention and/or treatment of inflammatory bowel diseases (IBDs)
A technology of Bacteroides fragilis and inflammatory bowel disease, applied in the field of microorganisms, can solve problems such as inflammatory bowel disease that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Cultivation of Bacteroides fragilis ZY-312
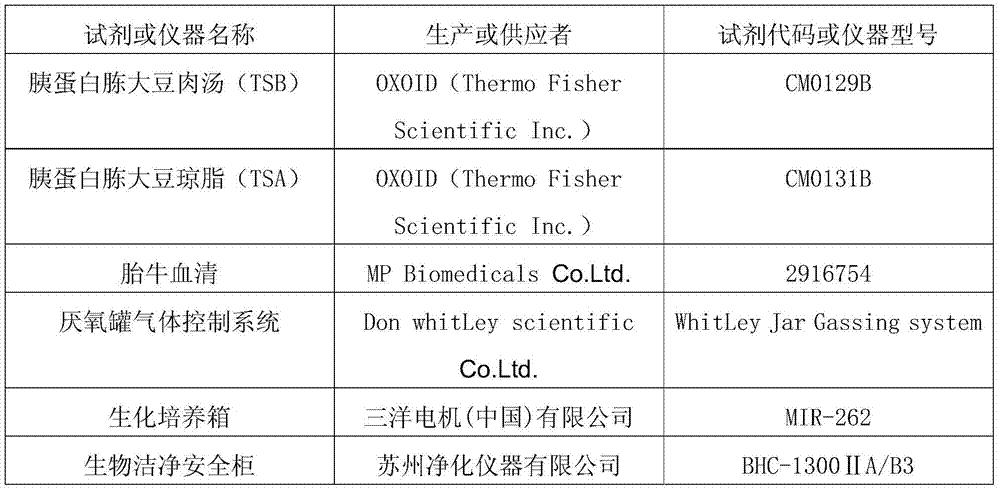
[0057] 1. The names of the reagents and instruments used are as follows:
[0058]
[0059] 2. Viable bacteria counting method
[0060] The count of live bacteria adopts the 10-fold serial dilution method:
[0061] Take 100 μL of the bacterial solution and add it to 900 μL of medium, and gradually dilute to an appropriate concentration. 4 concentration gradients were spotted on each plate, and each gradient was spotted 3 times, each spotting 20 μL. In a biochemical incubator at 37° C. for anaerobic culture for 48 hours, the number of colonies was counted (counted in a concentration gradient with the number of colonies ranging from 3 to 30).
[0062] The number of viable bacteria (CFU / mL) = the sum of three spotted colonies / 3 × 50 × dilution
[0063] 3. Training method
[0064] Step 1: Take a freeze-dried and preserved strain (ZY-312, Bd-312 or ATCC25285, the culture method is the same, so I will not list them one by ...
Embodiment 2
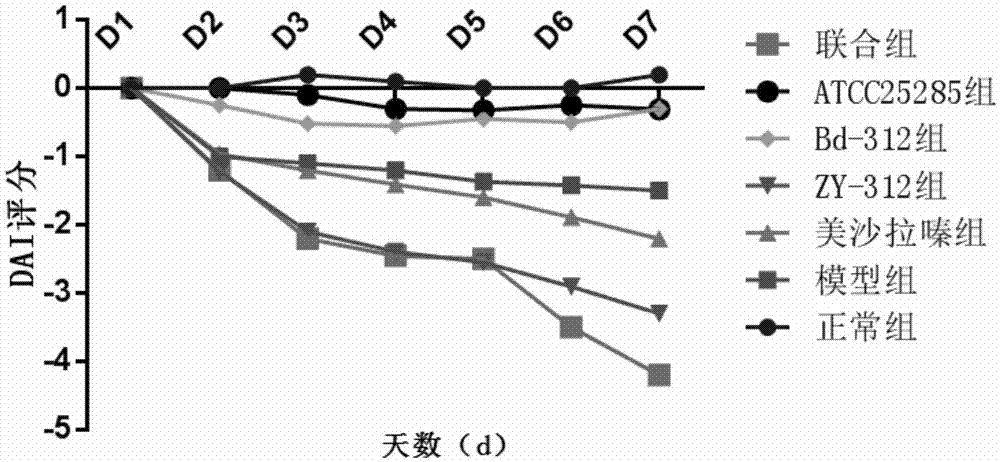
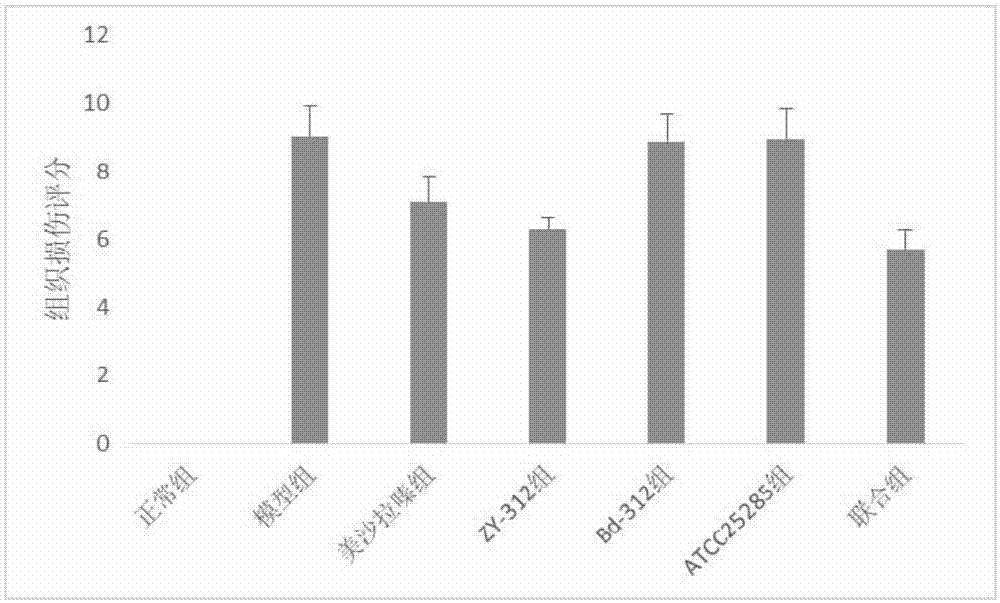
[0069] Effect of Bacteroides fragilis ZY-312 on experimental colitis in rats
[0070] Select 70 healthy and mature SD rats, half male and half male, body weight 200±20g, purchased from Guangdong Experimental Animal Center, raised in SPF grade animal room, randomly divided into 7 groups, 10 rats in each group, all rats before experiment Adapt to the environment for 1 week, and feed normal rat chow.
[0071] Using 5% DSS (dextransulfatesodium, DSS, purchased from Sigma, USA) solution to induce rat chronic ulcerative colitis model, 70 SD rats were randomly divided into the following 7 groups:
[0072] Normal control group, model group, mesalazine group, ZY-312 group, Bd-312 group, ATCC25285 group and combination group (ZY-312+mesalazine), the efficacy of each intervention group was detected by DAI score and tissue injury score . The animal grouping table is as follows:
[0073] Numbering
modeling
group
number of animals
[0074] A
no ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com