Amine compound and application thereof to antitumor drug
A technology of amine compounds and anti-tumor drugs, applied in the field of amine compounds and their application in anti-tumor drugs, can solve the problems of mortality, patient and family burden, and high difficulty of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
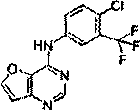
[0023] (1) Synthesis of 4-carbonylfuro[3,2-d]pyrimidine:
[0024]
[0025] Into a three-necked flask, add formamide (15 mL) and 2-carboxy-3-aminofuran (6.10 g, 48 mmol). The reaction mixture was then heated at 170 °C for 4-6 hours. The completion of the reaction was monitored by TLC; ice was added to the reaction mixture, resulting in a precipitate, which was filtered to obtain a solid, washed with water, redissolved in ethyl acetate, and dissolved in MgSO 4 After drying and concentration, 5.03 g of pure white solid furo-4-carbonyl[3,2-b]pyrimidine was obtained with a yield of 77%. 1 H-NMR (400 MHz, CDCl3) δ:7.09(d, 1H), 7.54(d, 1H), 7.89(s, 1H). 13 C-NMR (125 MHz, CDCl3) δ: 109.31, 120.49, 134.70, 143.12, 143.53, 151.34. LC-MS (ESI, pos, ion) m / z: 137[M+H].
[0026] (2) Synthesis of N-(4-chloro-3-(trifluoromethyl)phenyl)furo[3,2-d]pyrimidin-4-amine
[0027]
[0028] 5-Amino-2-chlorobenzotrifluoride (5.87g, 30mmol) was dissolved in methanol (50ml). Then 4-carbonylfu...
Embodiment 2
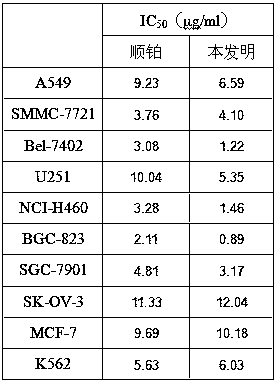
[0057] Example 2: Study on Bel-7402 Liver Cancer Transplanted Tumor Mouse Model
[0058] 1. Establishment of human liver cancer xenograft model in nude mice
[0059] SPF grade Balb / c nude mice, male, 4 weeks old, were transplanted into nude mice with human Bel-7402 liver cancer cells to form tumors, and then the nude mice were passaged for more than 2 generations. The tube needle was inoculated subcutaneously in the axilla of nude mice to establish a nude mouse xenograft cancer model of human liver cancer.
[0060] 2. Dosing regimen
[0061] Use a vernier caliper to measure the length and width of each animal tumor, according to the formula: V=1 / 2*ab 2 Calculate the tumor volume until the tumor volume grows to about 100mm 3 At the beginning of administration, all animals were randomly divided into model group, positive drug capecitabine group (400 mg / kg), high (100 mg / kg) and low (25 mg / kg) dose groups of the compound of the present invention according to tumor volume, with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com