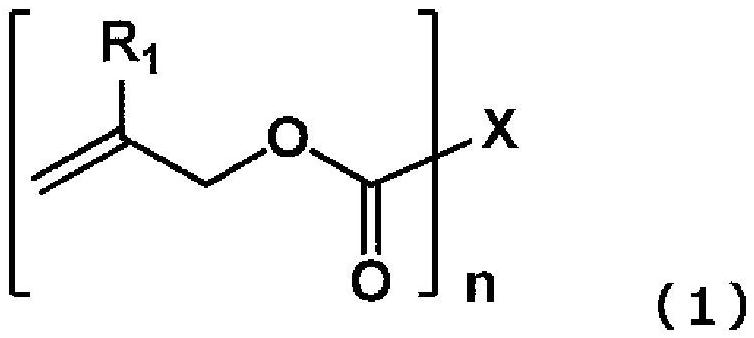
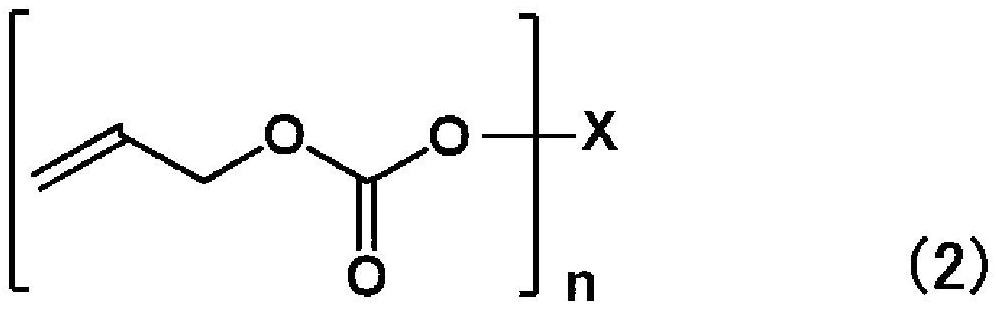
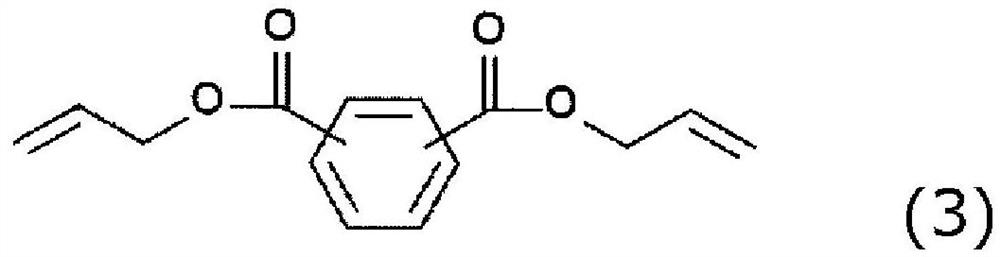
Polymerizable composition, method for producing organic glass using same, and organic glass
A technology of polymeric composition and polymeric compound, which is applied in applications, household appliances, and other household appliances, etc., and can solve problems such as optical distortion, decreased hardness of substrates, and decreased heat resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~9、 comparative example 1~6
[0347] The (A) radically polymerizable compound shown in Examples 1-9 of Table 1, and Comparative Examples 1-6, (C) photochromic compound, and other additive components were fully mixed at 70 degreeC. This liquid mixture was cooled to room temperature, (B) radical polymerization initiator was added, and it mixed at room temperature until it became uniform. Table 1 shows their compounding ratios.
[0348]The mixed solution containing the photochromic compound was poured into a mold formed by wrapping the outer peripheries of two disc-shaped glass plates with an adhesive tape, and casting polymerization was performed. During the polymerization, an air furnace was used, and the temperature was gradually raised to perform polymerization. After the polymerization, the casting mold was taken out from the air furnace, and after natural cooling, the solidified body was removed from the glass mold of the casting mold.
[0349] The resin yellowness index and photochromic properties of...
Embodiment 1
[0375] With respect to 99.1 parts by weight of commercially available poly(allyl carbonate) RAV 7NG (manufactured by ACOMON), 0.035 parts by weight of the photochromic compound Reversacol Heath Green (product name, manufactured by Vivimed Company), Reversacol Wembley Gray (Product name, manufactured by Vivimed Corporation) 0.065 parts by weight, 2-hydroxy-4-methoxybenzophenone (Product name, SEESORB 101, manufactured by SHIPRO KASEI Corporation) 0.15 parts by weight were dissolved, cooled to room temperature, and added as solidified 0.9 parts by weight of LUPEROX TAEC (product name, manufactured by Arkema Co., Ltd.) of the agent was injected into a mold formed by wrapping the outer peripheries of two disc-shaped glass plates with adhesive tape, and the temperature was gradually increased from room temperature to 110°C. 24 hours polymerization. Thereafter, the molded body was released from the mold and heated at 120° C. for 1 hour to perform post-polymerization to obtain a flat...
Embodiment 2
[0377] With respect to 99.1 parts by weight of commercially available poly(allyl carbonate) RAV 7NG (manufactured by ACOMON), 0.035 parts by weight of the photochromic compound Reversacol Heath Green (product name, manufactured by Vivimed Company), Reversacol Wembley Gray (product name, manufactured by Vivimed Corporation) 0.065 parts by weight, 2-hydroxy-4-methoxybenzophenone (product name: SEESORB 101, manufactured by SHIPRO KASEI Corporation) 0.15 parts by weight, 1,4-bis[(2, 6-diethyl-4-methylphenyl)amino]-9,10-anthraquinone (product name: Macrolex Blue RR, manufactured by LANXESS) 2.5ppm, 1,4-diamino-2,3-phenoxy Base-9, 10-anthraquinone (product name: Solvaperm Red Violet R, manufactured by CLARIANT Corporation) was dissolved at 2.5 ppm, and after cooling to room temperature, 0.9 parts by weight of LUPEROX TAEC (product name, manufactured by Arkema Corporation) was added as a curing agent, and injected Polymerization was performed for 24 hours while gradually raising the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com