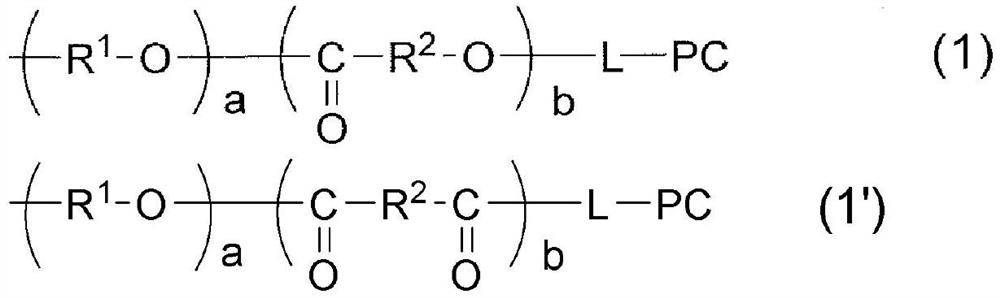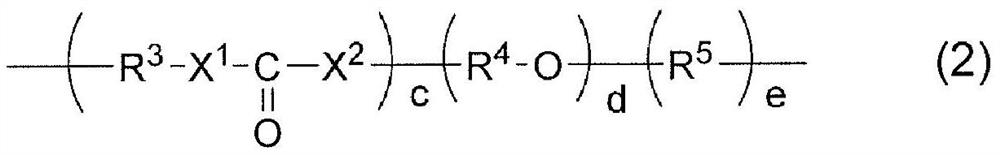Photochromic compound and curable composition containing said photochromic compound
A photochromic and compound technology, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, optics, etc., can solve the problems of dependence and difficulty in balancing, and achieve excellent color concentration and fading speed, and excellent photochromic properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
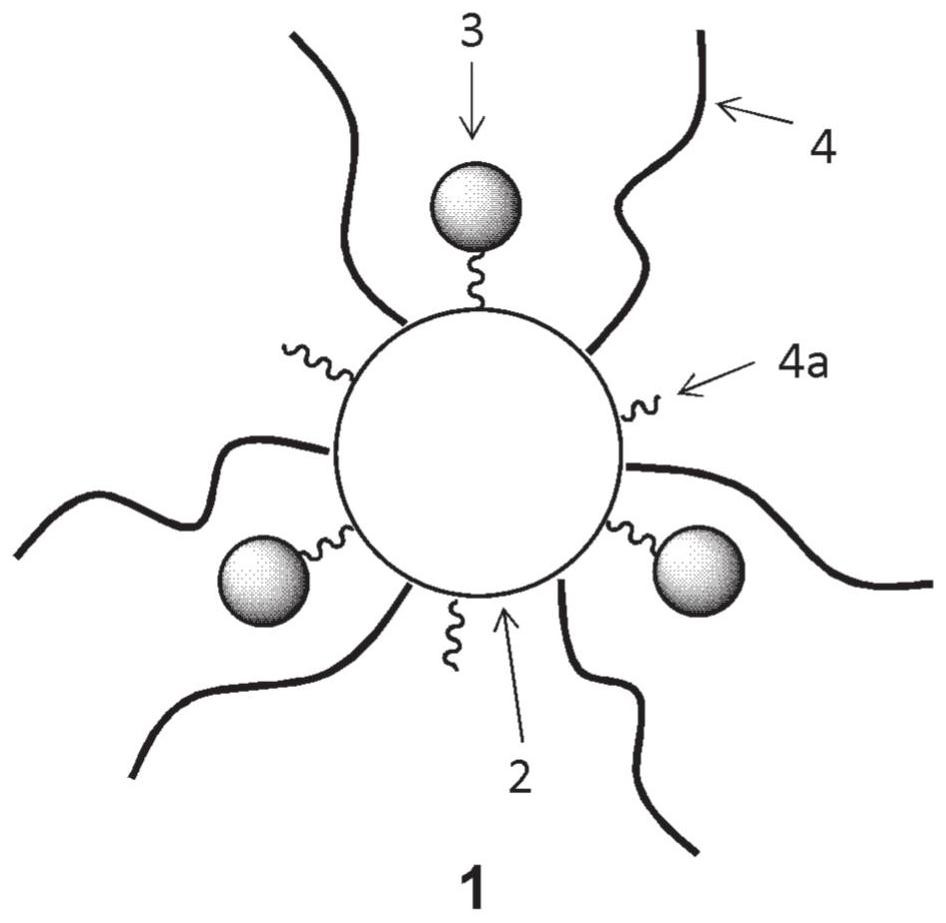
[0469] Synthesis of Cyclodextrin Conjugated Photochromic Compound (CyD1)
[0470] This CyD1 is a compound in which cyclodextrin is used as a compound forming a polyvalent residue.
[0471] first process
[0472] By the following formula (11)
[0473] [chemical 14]
[0474]
[0475] 4.7 g (10 mmol) of the compound represented, synthesized according to the method described in the International Publication No. 2006 / 022825 pamphlet, is obtained by the following formula (12)
[0476] [chemical 15]
[0477]
[0478] 100 mL of toluene was added to 5.3 g (15 mmol) of the indicated compound and 0.25 (1 mmol) of pyridinium p-toluenesulfonate, and heated and stirred at 75° C. for 1 hour. After cooling to room temperature, it was washed with 100 mL of water three times, and the organic layer was distilled off under reduced pressure. The residue obtained is purified by silica gel column chromatography, and obtained by the following formula (13):
[0479] [chemical 16]
[0480]...
Embodiment 2
[0515] Preparation of curable composition (Y1 (hereinafter sometimes abbreviated as (Y1))) and production and evaluation of photochromic cured product
[0516] (Preparation of curable composition)
[0517] According to the following formulation, each component was fully mixed, and the photochromic curable composition (Y1) was prepared.
[0518] formula;
[0519] (A) Photochromic compound
[0520] CyD1 (manufactured in Example 1): 69.3 mg (photochromic pigment: 9.6 μmol).
[0521] (B) Polymeric compound
[0522] (B1-1-1-4) norbornene methane diisocyanate: 4.58 g.
[0523] (B3-2) Pentaerythritol tetrakis(3-mercaptopropionate): 5.42 g.
[0524] (C) Polymerization curing accelerator
[0525] (C3-1) Dimethyl tin dichloride: 10 mg.
[0526] (Production and evaluation of photochromic cured product)
[0527] Using the above curable composition (Y1), a photochromic cured body was obtained by a kneading method.
[0528] Furthermore, the polymerizable compound (B) used in the ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com