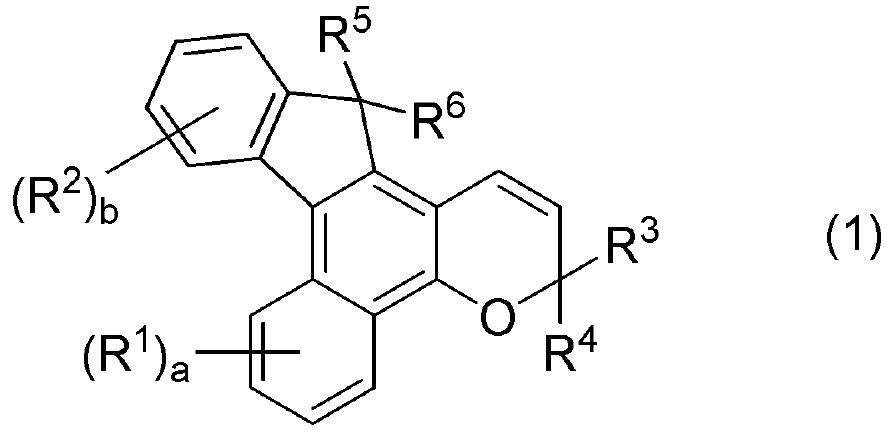
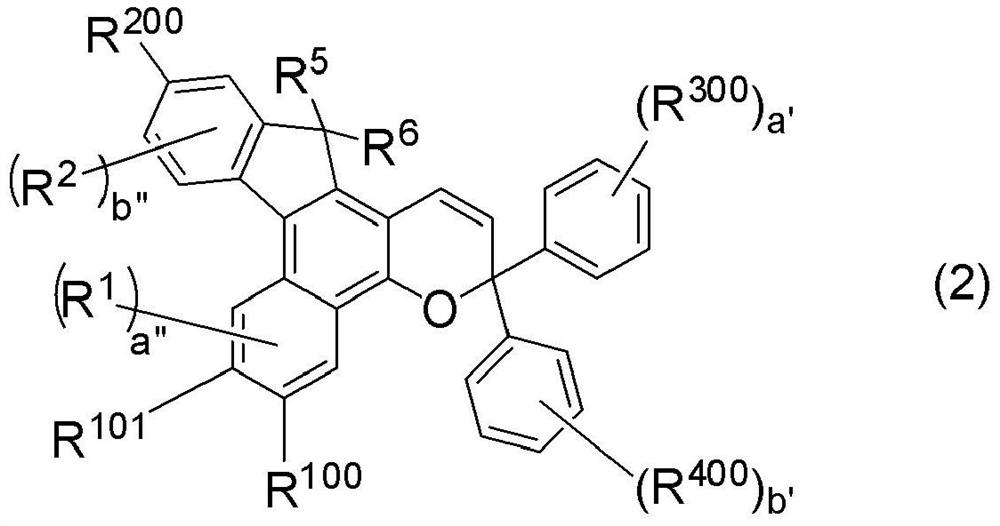
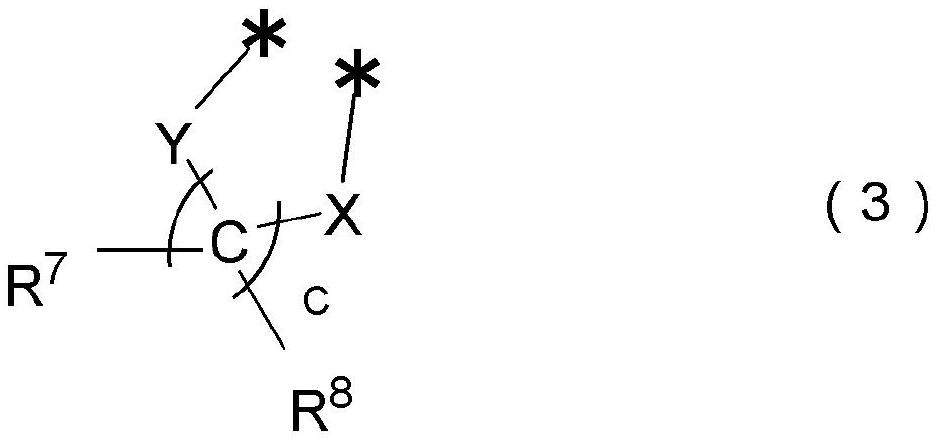
Photochromic compound, curable composition containing said photochromic compound, and optical article
A photochromic and compound technology, applied in the direction of color-changing fluorescent materials, optics, optical components, etc., can solve the problem of white turbidity of the cured body, and achieve the effect of good photochromic properties, mechanical properties, and excellent photochromic properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
[0563] Embodiment 1~5 (synthesis of photochromic compound of the present invention)
[0564]
[0565] 1st process
[0566] Polyethylene glycol monooleyl ether (36.7 g, 100 mmol) and tosyl chloride (21.0 g, 110 mmol) having a number average molecular weight of 357 were dissolved in pyridine (400 mL), followed by stirring. 1,4-Diazabicyclo[2.2.2]octane (2.2 g, 20 mmol) was dripped there, and it stirred at room temperature for 12 hours. After completion of the reaction, the reaction liquid was added to ice water, and extracted with dichloromethane. The extracted organic layer was washed with 10% hydrochloric acid, and then, the solvent was distilled off to obtain the following formula (16):
[0567] [chem 45]
[0568]
[0569] indicated compound.
[0570] 2nd process
[0571] 4-Hydroxybenzoic acid (6.2 g, 45 mmol), potassium carbonate (18.7 g, 135 mmol) were dissolved in DMF (450 mL). It heated while stirring so that the liquid temperature might become 80 degreeC. The...
Embodiment 2
[0615] 1st process
[0616] Except having used polypropylene glycol (number average molecular weight 2500) instead of the polypropylene glycol monobutyl ether (number average molecular weight 3500) used in the 8th step of Example 1, the reaction was carried out in the same manner as in the 8th step of Example 1, It is obtained by the following formula (26)
[0617] [Chemical 55]
[0618]
[0619] indicated compound.
[0620] 2nd process
[0621] In the 7th step of embodiment 1, in addition to replacing the naphthol compound of the above-mentioned formula (22), the following formula (27) is used:
[0622] [Chemical 56]
[0623]
[0624] Except the naphthol compound represented, carry out similarly, obtain by following formula (28)
[0625] [Chemical 57]
[0626]
[0627] Indicates the chromene precursor.
[0628] 3rd process
[0629] In the ninth step of Example 1, in addition to using the chromene precursor of the above formula (28) instead of the chromene com...
Embodiment 3
[0635] 1st process
[0636] 4,4-Dihydroxybenzophenone (1.0 g, 4.5 mmol), potassium carbonate (1.9 g, 13.5 mmol), and DMF (45 mL) were added, stirred, and heated until the internal temperature became 80°C. After heating, the compound (4.6 g, 9.1 mmol) of the above formula (16) was added dropwise over 1 hour. After dropping, reaction was performed at a liquid temperature of 80° C. for 4 hours. After the reaction, the reaction solution was cooled to room temperature, water was added, and liquid separation was performed using toluene. After the organic solvent was distilled off, the obtained residue was purified by silica gel column chromatography (developing solvent: chloroform-ethyl acetate mixed solvent) to obtain the following formula (30):
[0637] [Chemical 59]
[0638]
[0639] indicated compound.
[0640] 2nd process
[0641]Except that the compound represented by the above formula (30) was used instead of the compound represented by the above formula (20) used in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com