External skin preparation containing rivastigmine and pharmaceutical salt thereof and preparation method of external skin preparation
A technology for external preparations and medicinal salts, which is applied in the field of pharmaceutical preparations and can solve problems such as severe toxic and side effects of the gastrointestinal tract
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
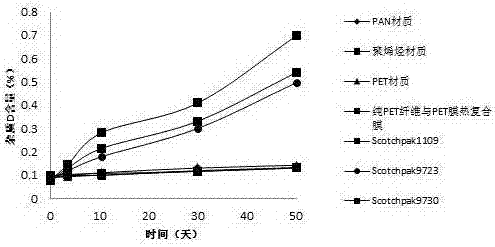
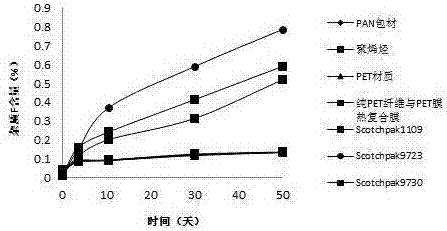
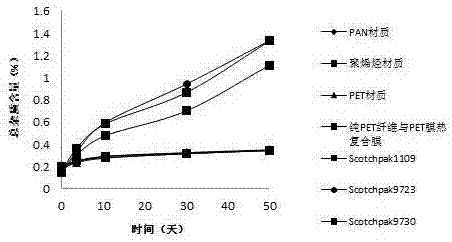
[0029] Backing layer: Thermal composite film of PET fiber and PET film
[0030] formula
[0031] Preparation method: import 15% silicone pressure-sensitive adhesive into a container, add isopropanol to stir, add formula amount of lactic acid, prepare colloid A, stir evenly, seal for use; add tocopherol to the remaining silicone pressure-sensitive adhesive 1. Stir the active ingredients of the prescription amount evenly; adjust the coating machine to coat, first coat with colloid A, and then continue to coat the drug-containing colloid; cut the coated sample into strips to the required size .
Embodiment 2
[0033] Backing layer: Thermal composite film of PET fiber and PET film
[0034] formula
[0035] Preparation method: the same as in Example 1.
Embodiment 3
[0037] Backing layer: Thermal composite film of PET fiber and PET film
[0038] formula
[0039] Preparation method: the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com