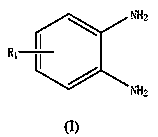
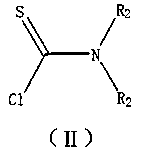
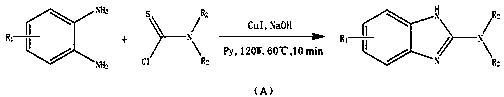A kind of method for the synthesis of benzimidazole derivatives catalyzed by N,N-dimethylaminothioformyl chloride derivatives under microwave radiation
A technology of dialkylaminothiocarbonyl chloride derivatives and microwave radiation is applied in the direction of organic chemistry and the like, which can solve the problems of difficulty in obtaining raw materials, severe reaction conditions, and high toxicity of reagents, and achieves environmental friendliness, simple operation and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: 2-(N,N-Dimethylamino)-benzimidazole: Add 1 mmol of o-phenylenediamine to the reaction vessel, add 0.05 mmol of cuprous iodide, 1 mmol of sodium hydroxide, N, N-dimethylaminothioformyl chloride 2.5 mmol, 3 ml pyridine. Placed in a microwave reactor and heated to 60 °C under 120 W power for continuous reaction for 10 min. After the reaction was completed, it was cooled to room temperature, concentrated under reduced pressure, and the product was purified by column chromatography to obtain a white solid with a yield of 88%.
Embodiment 2
[0032] Example 2: 2-(N,N-diethylamino)-benzimidazole: The preparation method is the same as in Example 1, adding 2.5 mmol of N,N-diethylaminothioformyl chloride to obtain a white solid with a yield of 86%.
Embodiment 3
[0033] Example 3: 2-(N,N-di-n-propylamino)-benzimidazole: The preparation method is the same as in Example 1, adding 2.5 mmol of N,N-di-n-propylaminothioformyl chloride to obtain a white solid. Yield 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com