Method for in vitro activating and amplifying natural killer cells
A cell and nuclear cell technology, applied in the field of biomedicine, can solve the problem of low overall process cost of NK cells, and achieve the effects of reducing the cost of in vitro expansion and culture, increasing the ratio and expansion multiple, large market prospects and economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 This example discloses a method for in vitro culture and expansion of natural killer cells (NK cells). The method (taking 50ml of human peripheral blood as an example) is as follows:
[0045] A. Activator Pretreatment
[0046] 1) Draw 500ul of activator, containing hyaluronic acid (concentration range between 0.10mg-10.00mg / ml) and CD16 antibody (concentration range between 0.5μg-2μg / ml), add to a 175cm 2 in a culture bottle. Add 20ml of PBS and mix thoroughly to disperse the liquid at the bottom of the bottle;
[0047] 2) Activate at 4°C for 12-16 hours; or store in a refrigerator at 4°C for 2 weeks.
[0048] 3) Aspirate off the activator, add physiological saline along the side wall of the culture bottle, shake the culture bottle gently to fully disperse the liquid.
[0049] 4) Aspirate off the normal saline, and the washed culture bottle should be used immediately.
[0050] B. Mononuclear cell isolation, plasma collection and processing
[0051] 1) Tur...
Embodiment 2
[0075] Example 2 This example discloses a method for in vitro culture and expansion of natural killer cells (NK cells). The method (taking 50ml of human umbilical cord blood as an example) is as follows:
[0076] A. Activator Pretreatment
[0077] 1) Draw 500u l of activator, containing hyaluronic acid (concentration range between 0.10mg-10.00mg / ml) and CD16 antibody (concentration range between 0.5μg-2μg / ml), add to a 175cm 2 in a culture bottle. Add 20ml of PBS and mix thoroughly to disperse the liquid at the bottom of the bottle.
[0078] 2) Activate at 4°C for 12-16 hours; or store in a refrigerator at 4°C for 2 weeks.
[0079] 3) Aspirate off the activator, add physiological saline along the side wall of the culture bottle, shake the culture bottle gently to fully disperse the liquid.
[0080] 4) Aspirate off the normal saline, and the washed culture bottle should be used immediately.
[0081] B. Mononuclear cell isolation, plasma collection and processing
[0082] 1...
experiment example 1
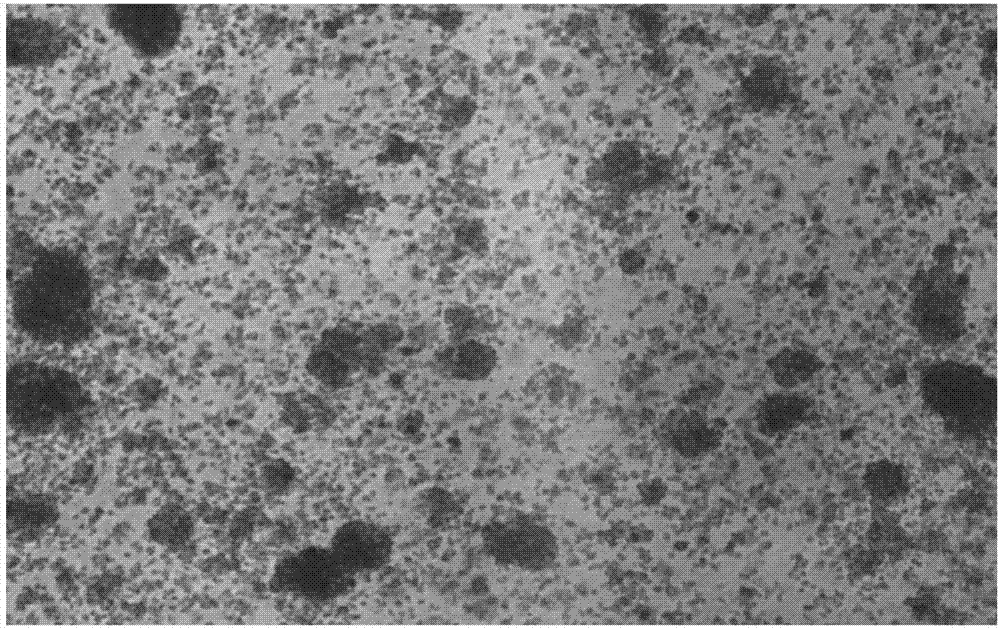
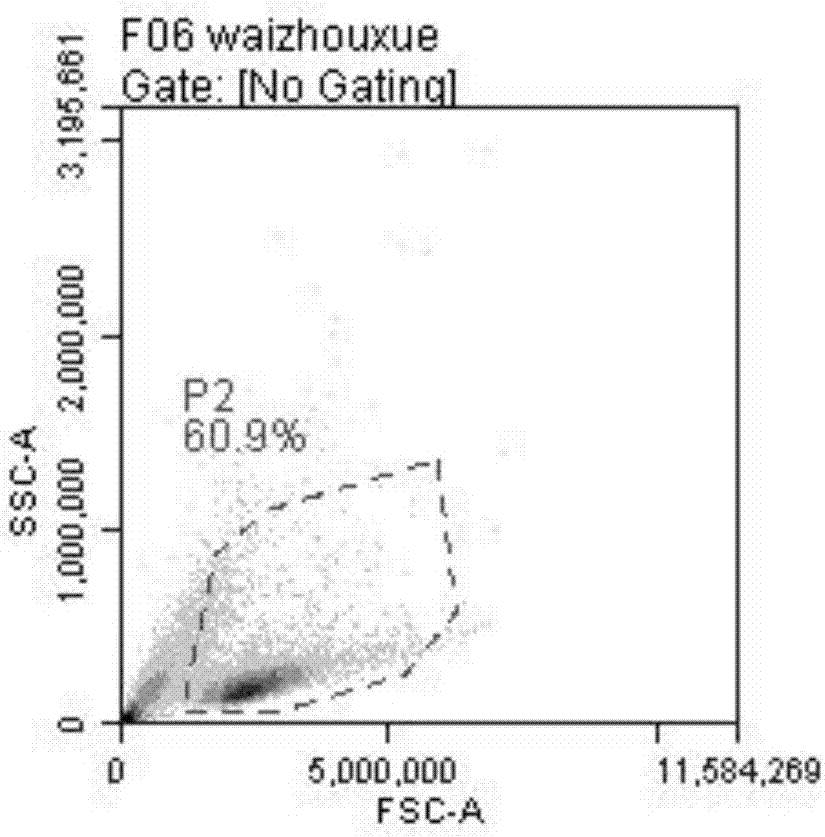
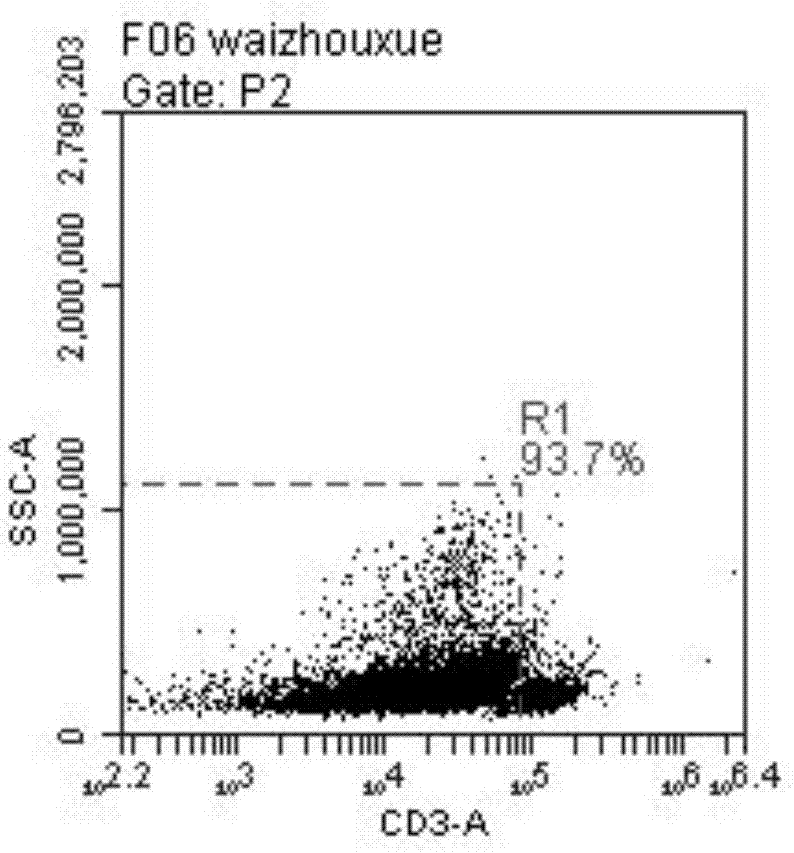
[0107] Experimental Example 1 The NK cells obtained in Example 1 were subjected to microscopic observation at the stage of induction and activation, and the results were as follows: figure 1 Significant NK cell colony formation is shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com