NOVEL FORMULATIONS OF PTHrP ANALOGUE
A technology of analogs and antimicrobial agents, applied in the field of treatment of osteoporosis to increase bone mass or increase bone mass, can solve the problems of dose range limitation, stimulation increase, etc., to achieve beneficial physiological effects increase, treatment duration decrease, eliminate The effect of adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
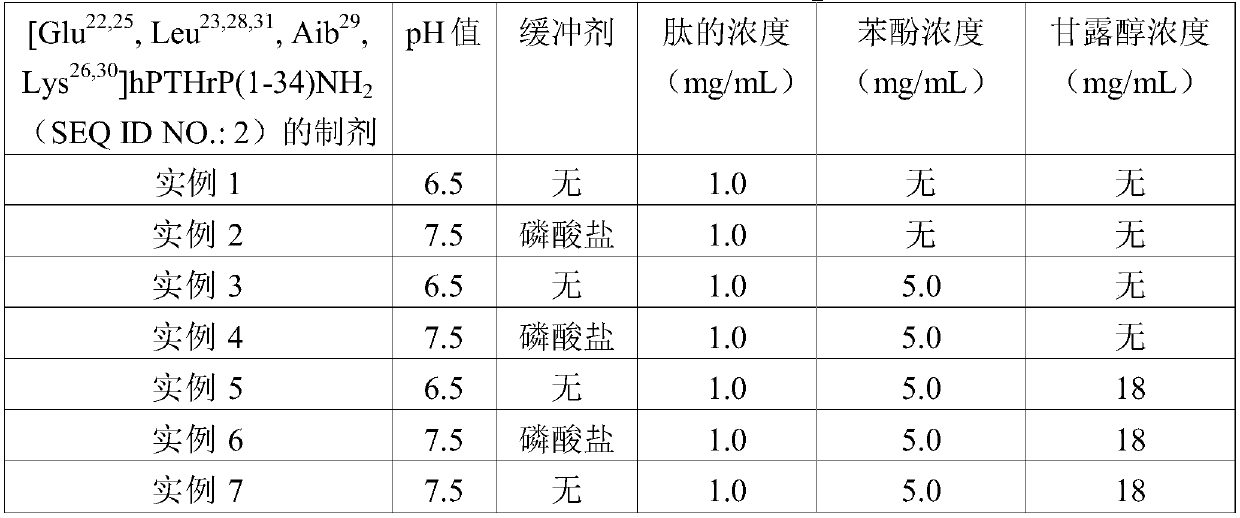
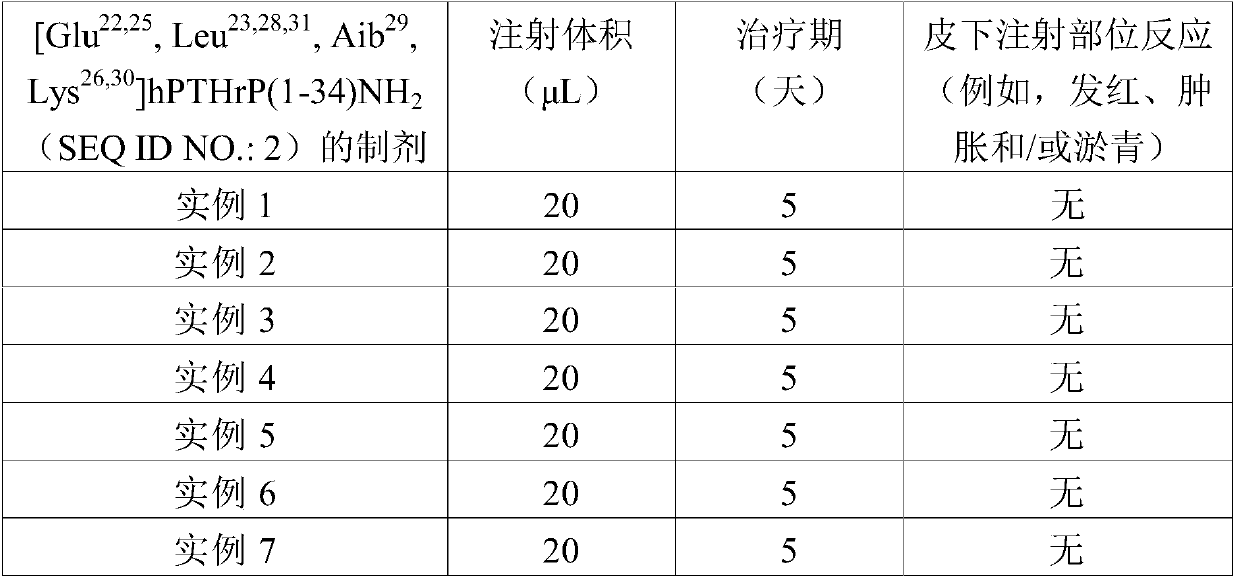
[0045] [Glu 22,25 , Leu 23,28,31 , Aib 29 ,Lvs 26,30 ]hPTHrP(1-34) NH 2 (SEQ ID NO.: 2) preparation
[0046] [Glu 22,25 ,Leu 23,28,31 , Aib 29 ,Lys 26,30 ]hPTHrP(1-34)NH 2 (SEQ ID NO.:2) 5.0 mg (free base) was dissolved in 4.50 mL of water for injection. The pH of the resulting solution was adjusted to 6.5 by using 1% hydrochloric acid solution and 1% sodium hydroxide solution, and the final volume of the solution was adjusted to 5.0 mL with water for injection.
example 2
[0048] [Glu in phosphate buffer at pH 7.5 22,25 , Leu 23,28,31 , Aib 29 ,Lvs 26,30 ]hPTHrP(1- 34) NH 2 (SEQ ID NO.: 2) preparation
[0049] [Glu 22,25 , Leu 23,28,31 , Aib 29 ,Lys 26,30 ]hPTHrP(1-34)NH 2 (SEQ ID NO.:2) 5.0 mg (free base) was dissolved in 5.0 mL of phosphate buffer (10 mM, Na 2 HPO 4 and NaH 2 PO 4 ). The resulting solution had a pH of 7.5.
example 3
[0051] [Glu 22,25 , Leu 23,28,31 , Aib 29 ,Lvs 26,30 ]hPTHrP(1-34)NH 2 (SEQ ID NO.: 2) preparation
[0052] [Glu 22,25 , Leu 23,28,31 , Aib 29 ,Lys 26,30 ]hPTHrP(1-34)NH 2 (SEQ ID NO.:2) 5.0 mg (free base) was dissolved in 4.60 mL water for injection containing 5.0 mg / mL phenol. The pH of the resulting solution was adjusted to 6.5 by using 1% hydrochloric acid solution and 1% sodium hydroxide solution, and the final volume of the solution was adjusted to 5.0 mL with water for injection containing phenol at a concentration of 5.0 mg / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap