A kind of docetaxel composite sustained release agent and preparation method thereof
A technology of docetaxel and sustained-release preparations, which is applied in the field of pharmaceutical anti-tumor drug preparations, can solve the problems of limited application, and achieve the effects of high drug loading, good stability and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The present invention relates to a kind of preparation method of docetaxel composite sustained-release agent, comprising the following steps:
[0027] S1: Dissolve docetaxel powder and lecithin in a tetrahydrofuran solvent, and react in a water bath at 45°C for 3 hours to obtain a docetaxel-phospholipid complex solution;
[0028] S2: removing aprotic tetrahydrofuran, which is easy to form complexes, in the docetaxel-phospholipid complex solution by rotary evaporation to obtain the docetaxel-phospholipid complex;
[0029] S3: putting the above docetaxel phospholipid complex into n-hexane solvent, and collecting the docetaxel phospholipid complex in n-hexane solvent by filtration;
[0030] S4: Remove the n-hexane in the docetaxel phospholipid complex by rotary evaporation (docetaxel is insoluble in n-hexane), and obtain a pale yellow docetaxel phospholipid complex by drying;
[0031] S5: putting the above light yellow docetaxel phospholipid complex, cholesterol, and polo...
Embodiment 1
[0046] A preparation method of docetaxel composite slow-release agent, comprising the following steps:
[0047] S1: dissolving docetaxel powder and soybean lecithin (mass ratio: 1:5) in an aprotic tetrahydrofuran solvent that is easy to form a complex, and reacting in a water bath at 45°C for 3 hours to obtain a docetaxel-phospholipid complex solution;
[0048] S2: removing tetrahydrofuran in the docetaxel-phospholipid complex solution by rotary evaporation to obtain the docetaxel-phospholipid complex;
[0049] S3: putting the above docetaxel phospholipid complex into n-hexane solvent, and collecting the docetaxel phospholipid complex in n-hexane solvent by filtration;
[0050] S4: remove n-hexane in the docetaxel phospholipid complex by rotary evaporation, and obtain a light yellow docetaxel phospholipid complex by drying;
[0051] S5: Put the above light yellow docetaxel phospholipid complex, cholesterol and poloxamer into tetrahydrofuran to obtain an organic solution conta...
Embodiment 2
[0057] A method for preparing the docetaxel composite slow-release preparation as described in Example 1, wherein the mass ratio of docetaxel powder and soybean lecithin in S1 is: 1:4.
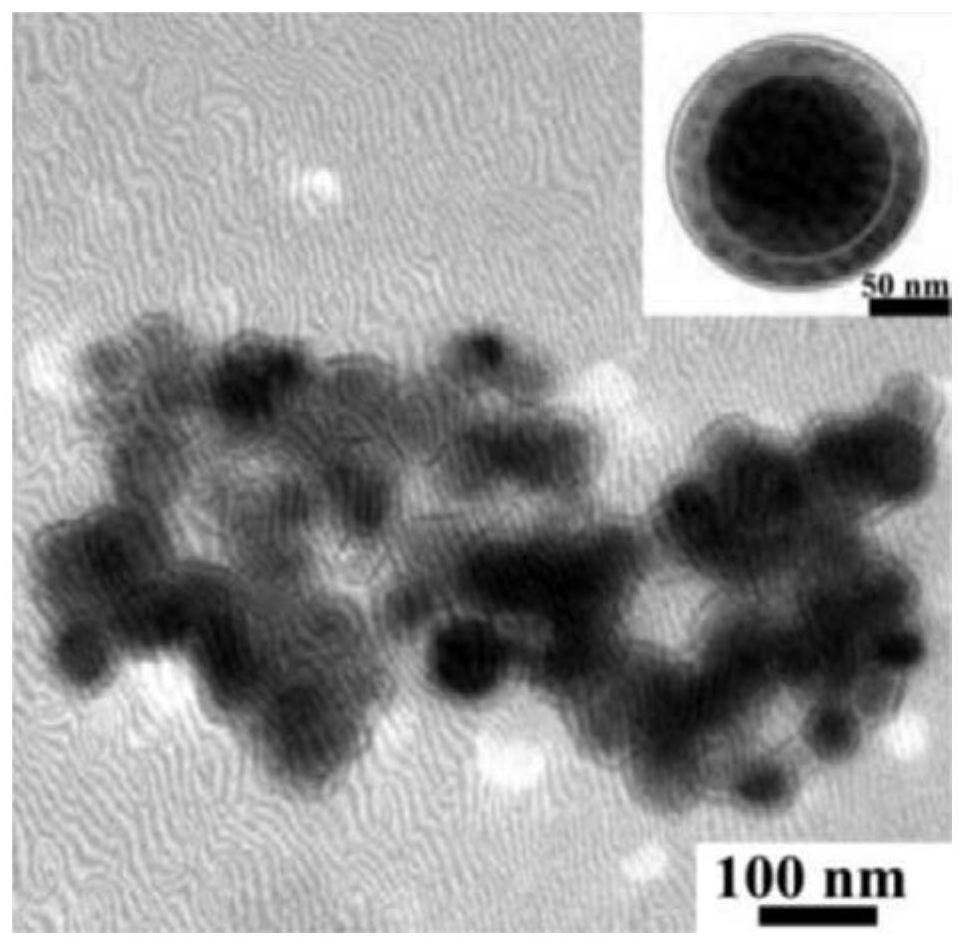
[0058] The average particle size of the docetaxel composite slow-release agent prepared by this method is about 130nm, and the drug loading is 14.42%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com