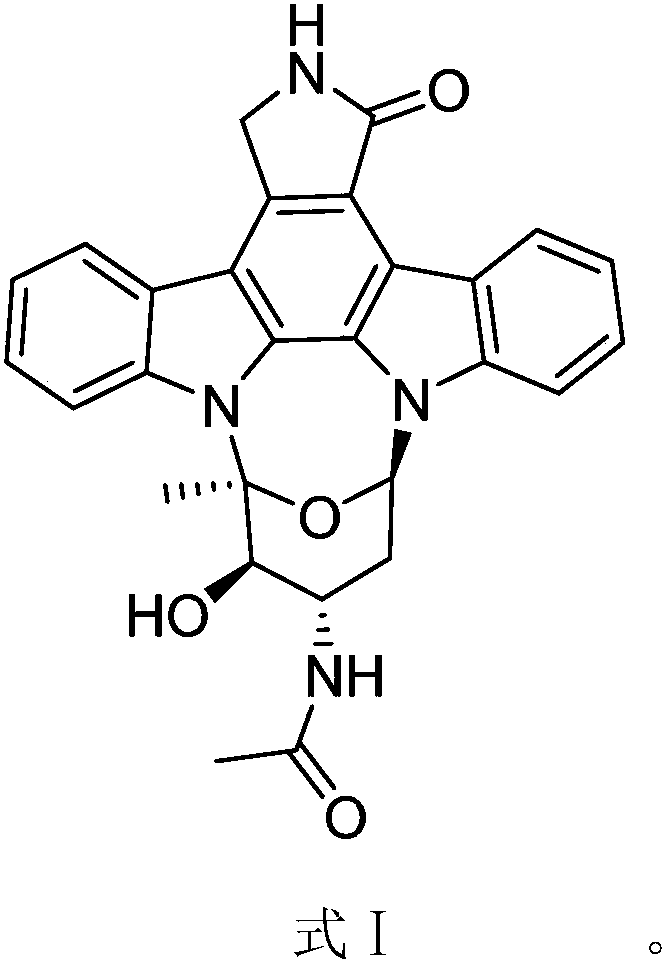
3-O-demethylation-4-N-demethylation-4-N-ethanoyl-staurosporine and preparation method and application thereof
A staurosporine and demethylation technology, applied in the field of actinomycete culture and preparation of compounds, can solve the problems of limited research, poor protein kinase selectivity, etc., and achieve strong cytotoxicity, strong anti-tumor activity, and good protein kinase inhibitory activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Fermentation of compounds
[0024] The actinomycete adopts the streptomyces Streptomycessp.CICC 11026 sold by China Industrial Microorganism Culture Collection Management Center;
[0025] 1) Inoculate Actinomyces Streptomyces sp.CICC 11026 into 500mL Erlenmeyer flasks, each bottle contains 250mL Gaoshi No. 1 liquid medium, and cultivate them in a shaker at 28°C and 180rpm for 3 days to obtain a fermentable culture the seed liquid;
[0026] 2) Inoculate the seed solution obtained in step 1) into rice medium (rice medium, made of the following components: 40g rice, 60mL water, 1.5g sea salt), the inoculation volume is 12mL, and the culture condition is constant temperature at 28°C Static culture for 70 days to obtain a solid fermentation product containing the compound with anti-tumor activity of the present invention.
[0027] 2. Compound preparation and identification
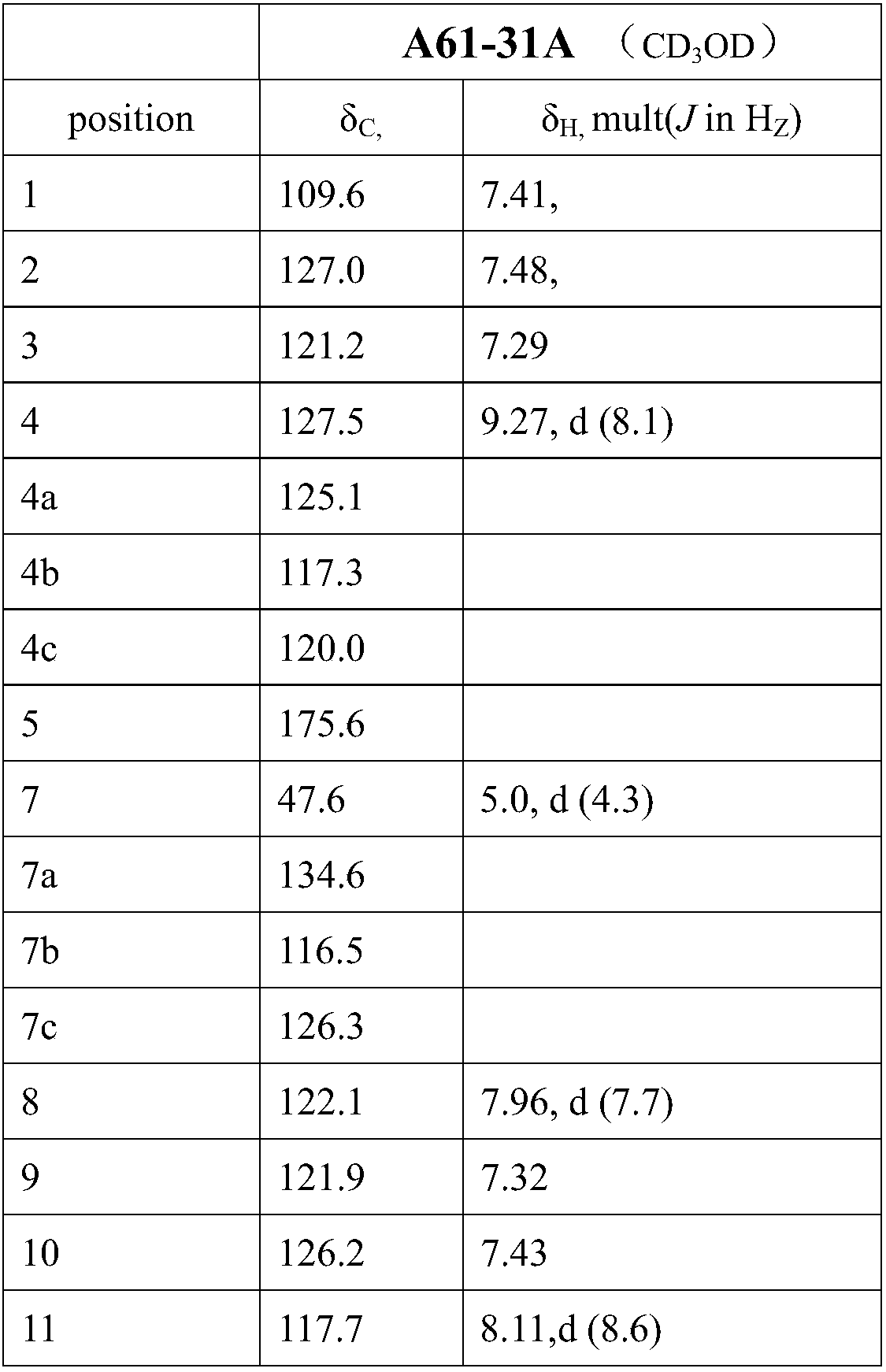
[0028] The solid fermentation product containing the compound with antitumor activity of the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com