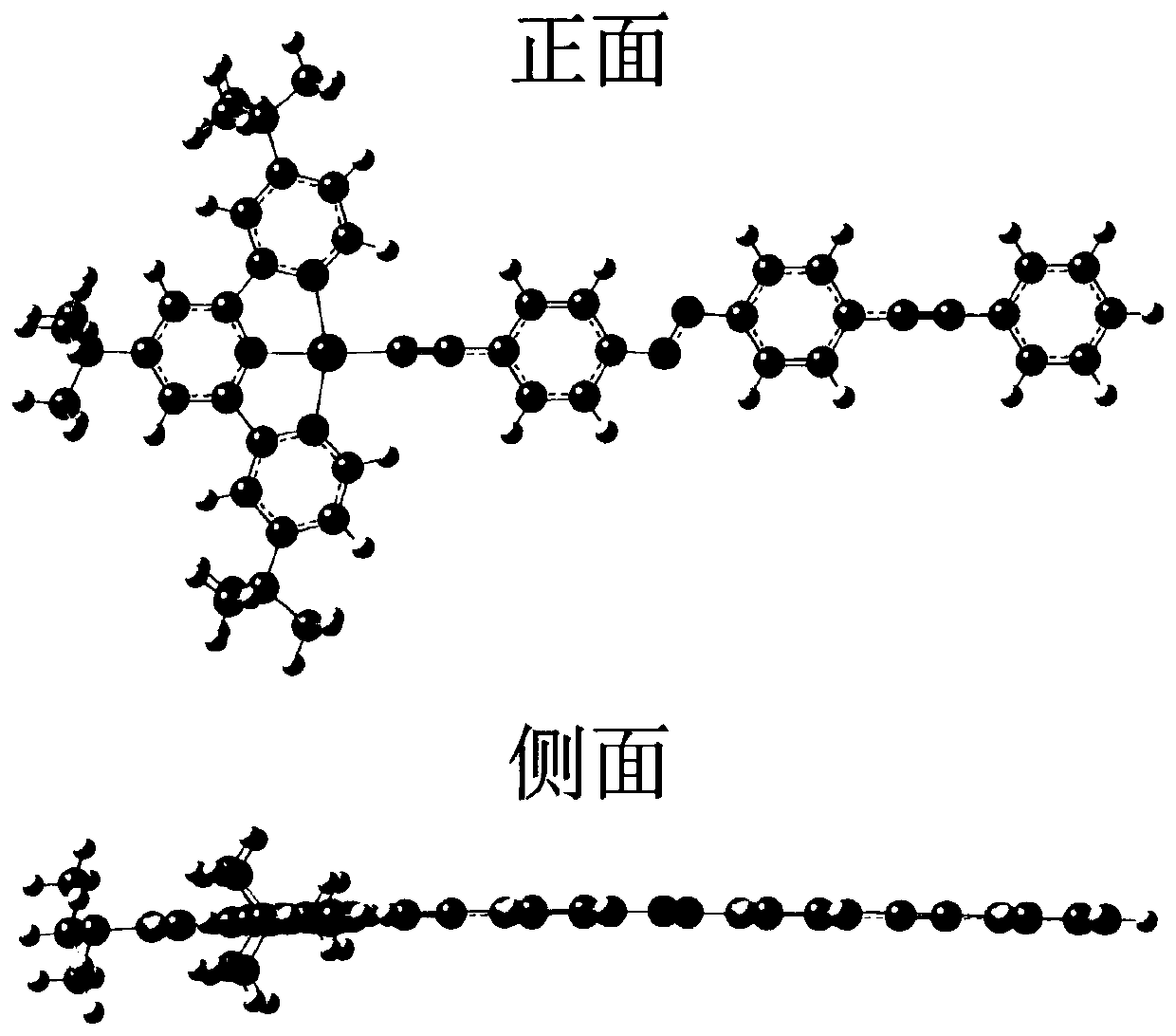
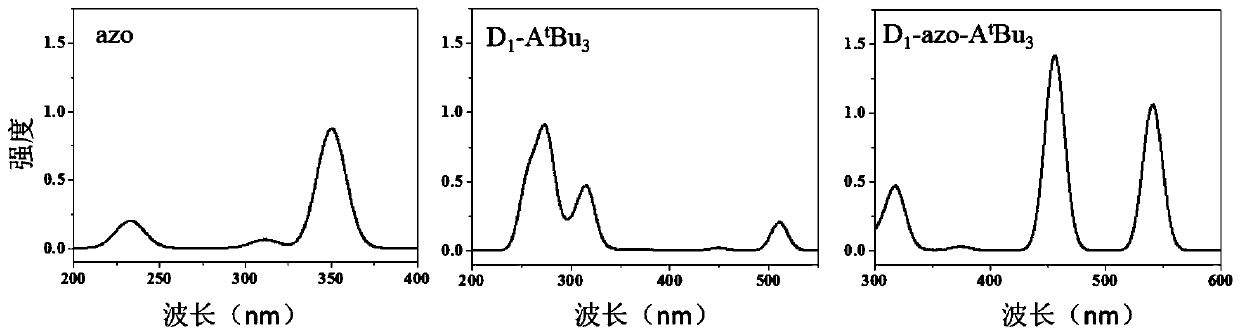
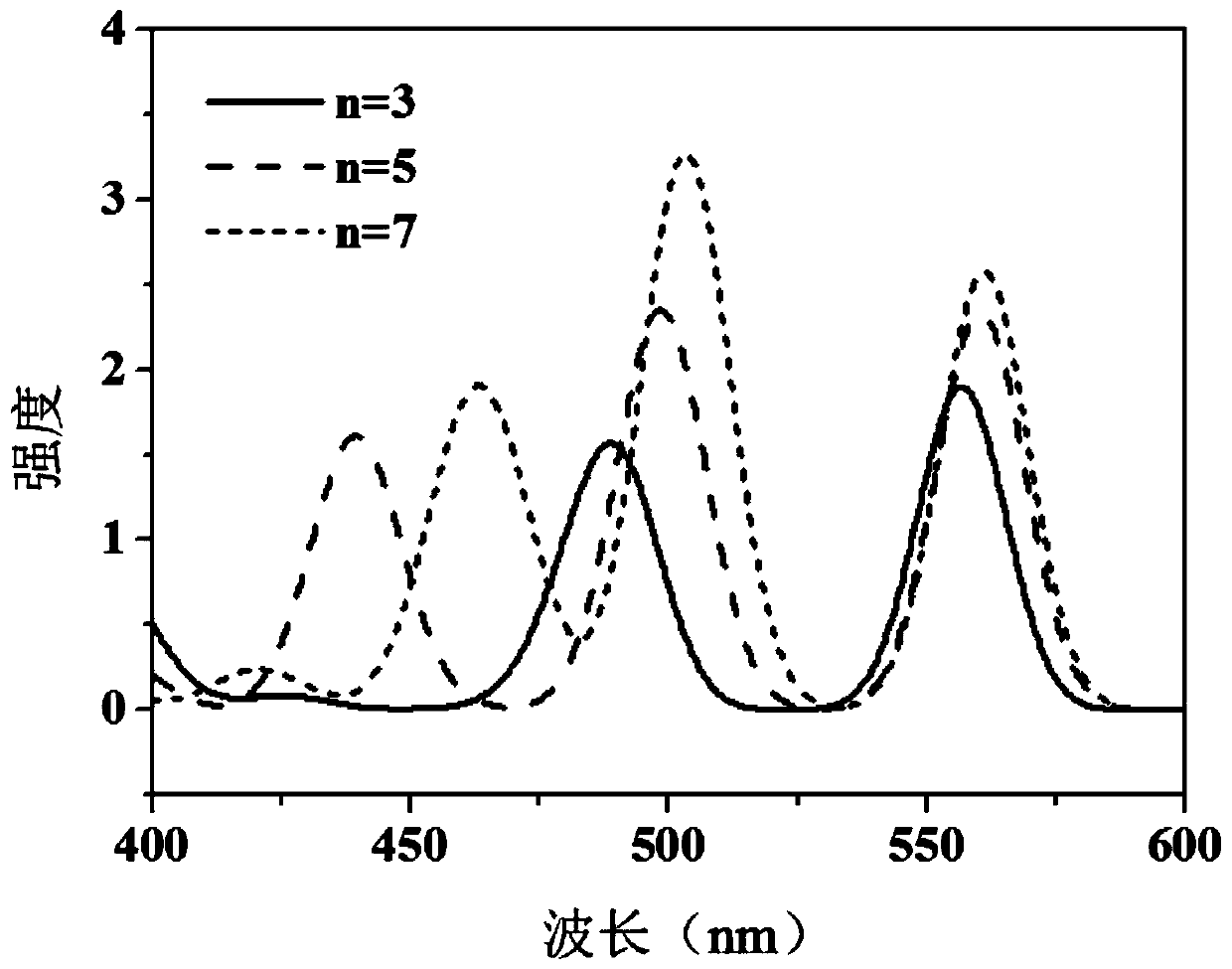
A self-regulated photoelectric conversion molecule and its preparation method
A photoelectric conversion and molecular technology, applied in photovoltaic power generation, chemical instruments and methods, circuits, etc., can solve the problems of reduced photoelectric conversion efficiency and inability to switch conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Preparation of 4,4'-diiodoazobenzene (I-azo-I):
[0099] (1) Use aniline as a precursor, put it into a four-necked flask, and after fully stirring, slowly add powdered iodine in batches, finish adding within 30 minutes, and continue stirring for 30 minutes until the reaction ends;
[0100] (2) Add the reaction solution to saturated NaHSO 3 Stir in the solution, filter with suction, and recrystallize the obtained solid with petroleum ether to obtain p-iodoaniline crystals;
[0101] (3) Add p-iodoaniline, potassium permanganate, copper sulfate pentahydrate and chloroform in the round bottom flask, stir and reflux for 48h, until the reaction is finished;
[0102] (4) Filtration, the reaction residue was washed with saturated sodium thiosulfate solution, brine and water successively, the solvent was evaporated under reduced pressure, and then separated and purified by column chromatography (eluent: sherwood oil / ethyl acetate=6 / 1 ) to obtain 4,4'-diiodoazobenzene as a soli...
Embodiment 2
[0104] Preparation of phenylacetylene (D 1 ):
[0105] (1) Add styrene and CCl in the three-necked flask 4 , stir evenly, cool to 5~15°C, slowly drop Br under stirring 2 CCl 4 Solution, continue to stir and react for 30-60min, filter to obtain dibromophenylethane solid;
[0106] (2) Dibromophenylethane solid, KOH and CH 3 OH was added to the three-necked flask, heated to reflux for 1-2 hours, cooled, filtered, the filtrate was extracted with ether, the upper layer solution was collected, subjected to atmospheric distillation, and then purified by vacuum distillation to obtain phenylacetylene liquid.
Embodiment 3
[0108] Preparation of 4-phenyl-2,2’,6’,2”-terpyridine platinum chloride complex (APh-Cl):
[0109] (1) Dissolve diacetylpyridine and benzaldehyde in ethanol, then add NaOH solution, stir for 30min, add the ethanol solution of ammonium acetate, stir at normal temperature for 3d, solids are produced, add a large amount of water until no precipitation, Suction filtration, the filter cake was washed with dichloromethane and saturated sodium bicarbonate respectively, dried with anhydrous magnesium sulfate, and then recrystallized with ethanol to obtain 4-phenyl terpyridine crystals;
[0110] (2) Dissolve 4-phenyl terpyridine crystals in hot acetonitrile, and slowly add K thereto 2 PtCl 4 Aqueous solution, heating, reflux reaction 24h, until the end of the reaction;
[0111] (3) After the reaction solution was cooled, it was suction filtered, and the filter cake was washed with water, dichloromethane, and ether in sequence, and dried to obtain a solid of 4-phenyl-2,2',6',2"-terpyr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com