Synthesis method of pomalidomide
A technology of pomalidomide and its synthetic method, which is applied in the field of medicinal chemistry, can solve the problems of high cost of reaction reagents, harsh conditions, and large pollution, and achieve the effects of reducing production costs and environmental pollution, mild reaction conditions, and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
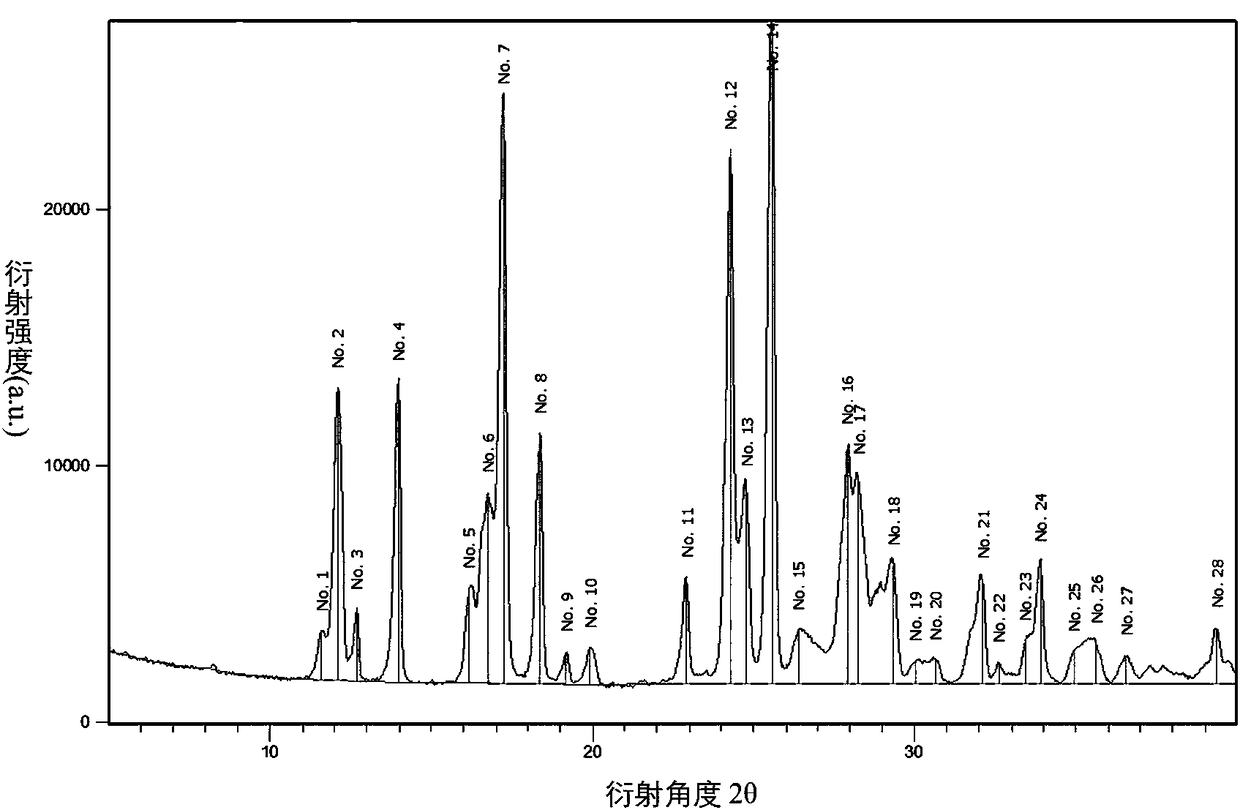
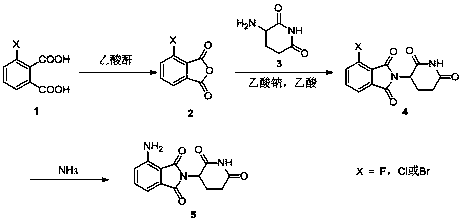
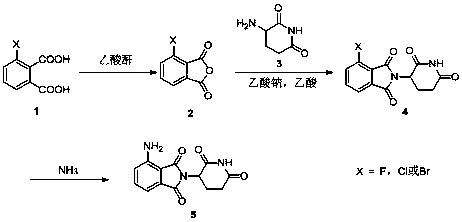
Image
Examples
Embodiment 1
[0027] Add 60 g of 3-bromophthalic acid and 60 mL of acetic anhydride into a 500 mL three-neck flask, stir at 80°C for 3 h, spot the plate to confirm that the reaction is complete, cool to room temperature, filter, and wash with 2×30 mL of petroleum ether. Dry 3-bromophthalic anhydride, yield 89%.
[0028] Add 43.4 g of 3-bromophthalic anhydride into a 1 L single-necked bottle, add 300 mL of acetic acid and stir, then add 36.7 g of 3-amino-2,6-piperidinedione hydrochloride and 19.8 g of sodium acetate in sequence Reflux for 3 h, cool to room temperature, filter, wash with 2×50 mL of water and 30 mL of ethanol, filter and dry to obtain compound 4 with a yield of 94.1%.
[0029] 64.1 g of compound 4, 500 mL of acetonitrile, 18 mL of ammonia water, 40 g of potassium carbonate and 0.5 g of cuprous oxide were reacted in a 1 L closed system at 50°C for 6 hours. After the reaction was completed, the solid was removed by filtration and evaporated to dryness to obtain the crude produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com