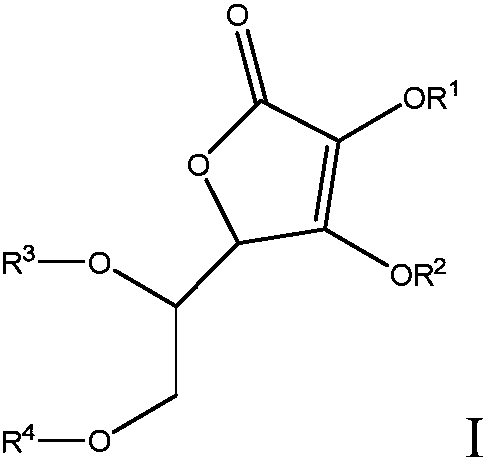
Redox polymerizable composition with photolabile reducing agents
A polymeric composition, photo-instability technology, applied in organic chemistry, chemical/physical process, catalyst activation/preparation, etc., can solve problems such as stress accumulation, poor storage stability of formulations, premature curing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0288] All amounts and percentages are by weight unless otherwise indicated.
[0289] materials used :
[0290] Acetone (EMD Millipore Corporation, Billerica, MA)
[0291] Acm: Acrylamide (Zibo Xinye Chemical Company, Zibo, China)
[0292] Ammonium chloride (EMD Chemicals, Inc. Gibbstown, NJ)
[0293] L-Ascorbic acid (Alfa Aesar, Ward Hill, MA)
[0294] L-Ascorbic acid-6-palmitate (Alfa Aesar, Ward Hill, MA)
[0295] BA: n-butyl acrylate (BASF Corporation, Florham Park, NJ)
[0296] BAYMOD 35.42 (Lanxess, Cologne, Germany)
[0297] BDGMA: Butyldiethylene glycol methacrylate (Evonik Industries, Marl, Germany)
[0298] BENZOFLEX 9-88, Plasticizer (Eastman Chemical Co., Kingsport, TN)
[0299] Benzyltributylammonium chloride (Sigma-Aldrich, St. Louis, MO)
[0300] BisGMA: 2,2-bis[4-hydroxy-3-methacryloyloxy)propoxyphenyl]propane (Sigma Aldrich, St.Louis, MO) )
[0301] 4-Bromomethyl-6,7-dimethoxycoumarin (Sigma-Aldrich, St. Louis, MO)
[0302] CAB-O-SIL TS720 (Cabot Co...
preparation example 1
[0375] Preparation Example 1 (PE-1): Synthesis of 5,6-O-isopropylidene-L-ascorbic acid (p-AA)
[0376] The material was prepared according to previous literature (Bioorg. Med. Chem. 2003, vol. 11, 827 ("Bioorganic Medical Chemistry", 2003, Vol. 11, p. 827)). To a suspension of L-ascorbic acid (20.0 g, 114 mmol) in acetone (200 mL) was added 2,2-dimethoxypropane (20.4 g, 196 mmol) and 10-camphorsulfonic acid (1.32 g, 5.68 mmol). The resulting mixture was stirred overnight at room temperature. To the resulting slurry was added about 0.6 g of triethylamine. A portion of hexane was added to the mixture and the white precipitate was collected via vacuum filtration and washed with additional hexane. The material was dried under vacuum to afford the desired product (21.0 g, 86% yield). 1 H NMR was consistent with the desired product.
[0377]
preparation example 2
[0378] Preparation Example 2 (PE-2): Synthesis of nitrobenzyl-protected p-AA
[0379] Potassium carbonate (3.03 g, 21.9 mmol) was added to a solution of p-AA (4.73 g, 21.9 mmol) in 40 ml 1:1 THF / DMSO. The resulting mixture was allowed to stir for 30 min. A solution of 2-nitrobenzyl bromide (4.73 g, 21.9 mmol) in 20 mL of 1:1 THF / DMSO was then added dropwise via addition funnel over 10 min. The resulting mixture was allowed to stir overnight under nitrogen atmosphere, during which time it turned dark orange. After removing THF under reduced pressure, about 200 mL of H 2 O was added to the mixture, then extracted with EtOAc (3x). The combined organic layers were washed with H 2 O(3x) and saturated aqueous NaCl, washed with MgSO 4Dry, filter, and concentrate to a yellow solid. This material was purified via trituration with 2:1 hexanes / EtOAc to afford 4.47 g of the product (58% yield) as a pale yellow solid. 1 H NMR was consistent with the desired product.
[0380]
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| lap shear strength | aaaaa | aaaaa |
| shear strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap