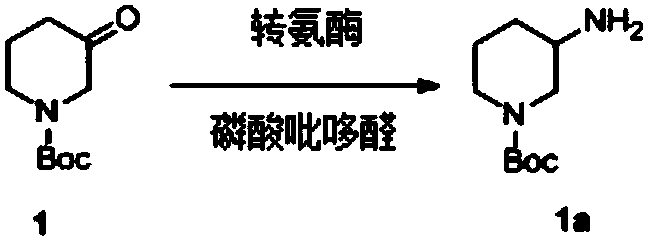
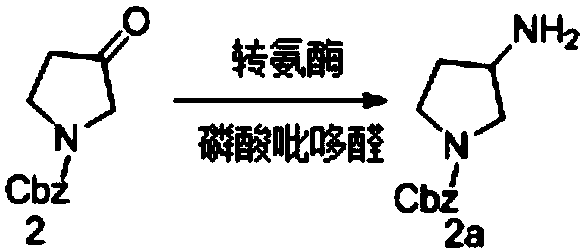
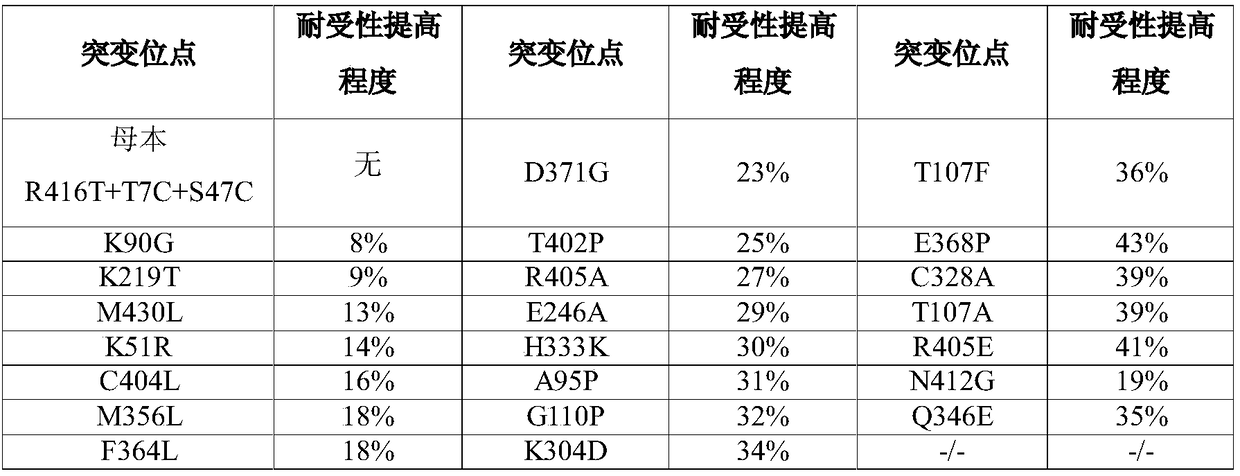
Transaminase mutant and applications thereof
A technology of transaminase and mutant, applied in the field of enzyme engineering, can solve the problems of limited application, poor tolerance of transaminase activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133]Example 1: Mutant 30°C, pH 9.5, 50% DMSO tolerance detection
[0134] The crude enzyme was treated at 30° C., pH 9.5, and DMSO concentration was 50% for 1 h, then in a 10 mL reaction flask, 0.1 g of substrate 1 was added, and 4 eq of isopropylamine hydrochloride and 0.6-1 mg of PLP ( 5'-pyridoxal phosphate), and then add 5 mg of the above-mentioned treated enzyme, and stir for 16 hours at 30° C., pH 9.5, and 50% DMSO concentration. The conversion rate of the system was detected by HPLC, and the mutant reaction data are shown in Table 12.
[0135] Table 12:
[0136]
Embodiment 2
[0137] Example 2: Mutant 45°C, pH 10, 50% DMSO tolerance test
[0138] Crude enzyme was treated at 45°C, pH 10, DMSO concentration was 50% environment for 1h, then in a 10mL reaction flask, 0.1g substrate 1 was added, and 4eq isopropylamine hydrochloride and 0.6-1mgPLP (5'- pyridoxal phosphate), and then add 5 mg of the enzyme after the above treatment, and stir for 16 hours at 45° C., pH 10, and DMSO concentration of 50% in an environment with constant temperature. The conversion rate of the system was detected by HPLC, and the mutant reaction data are shown in Table 13.
[0139] Table 13:
[0140]
[0141]
Embodiment 3
[0142] Example 3: Mutant 30°C, pH8, 35% Methanol Tolerance Detection
[0143] Treat the crude enzyme at 30°C, pH8, 35% MeOH concentration environment for 1h, then add 0.1g substrate 1, 4eq isopropylamine hydrochloride and 0.6-1mgPLP (5'- pyridoxal phosphate), and then add 5 mg of the enzyme after the above treatment, and continue stirring at 30° C., pH 8, and 35% MeOH concentration environment for 16 hours. The conversion rate of the system was detected by HPLC, and the mutant reaction data are shown in Table 14.
[0144] Table 14:
[0145]
[0146]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com