Application of icarisid II or medical carrier of icarisid II to prevention and/or treatment of erectile dysfunction
A technology for erectile dysfunction and icariin, which is applied in the field of medicine, can solve the problems of insignificant improvement of pathological changes in an animal model of neurogenic ED, and achieve a remarkable effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1) Experimental method:
[0054] (1) Experimental animals and groups
[0055] Ninety-six newborn 1-day-old male SD rats.
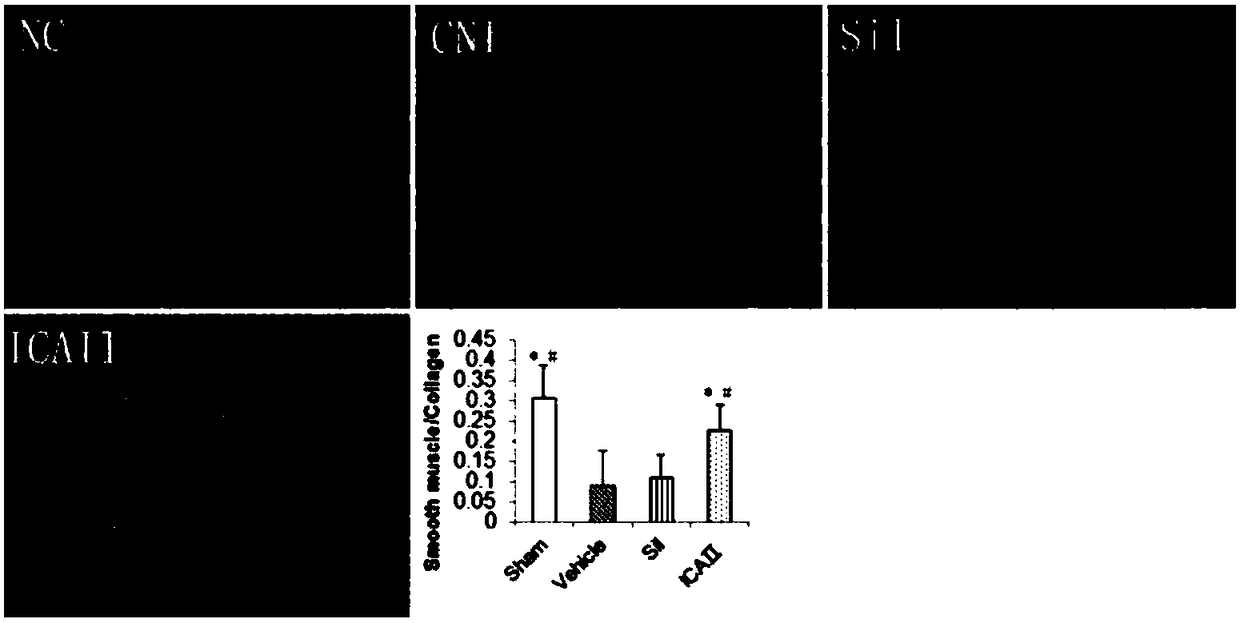
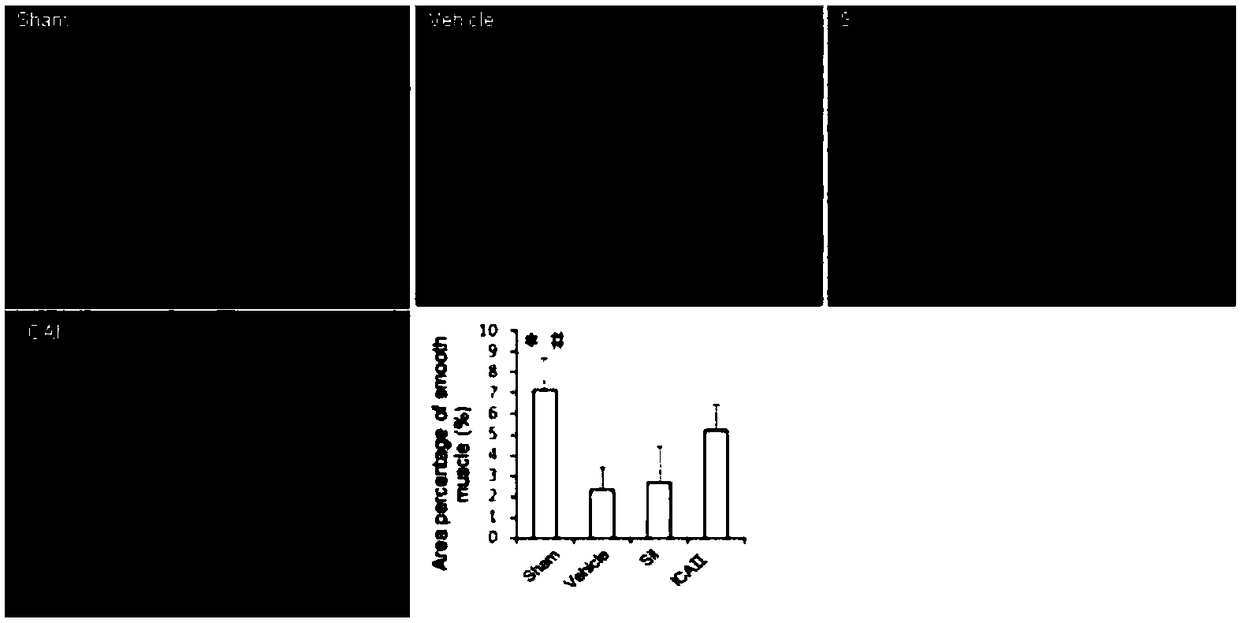
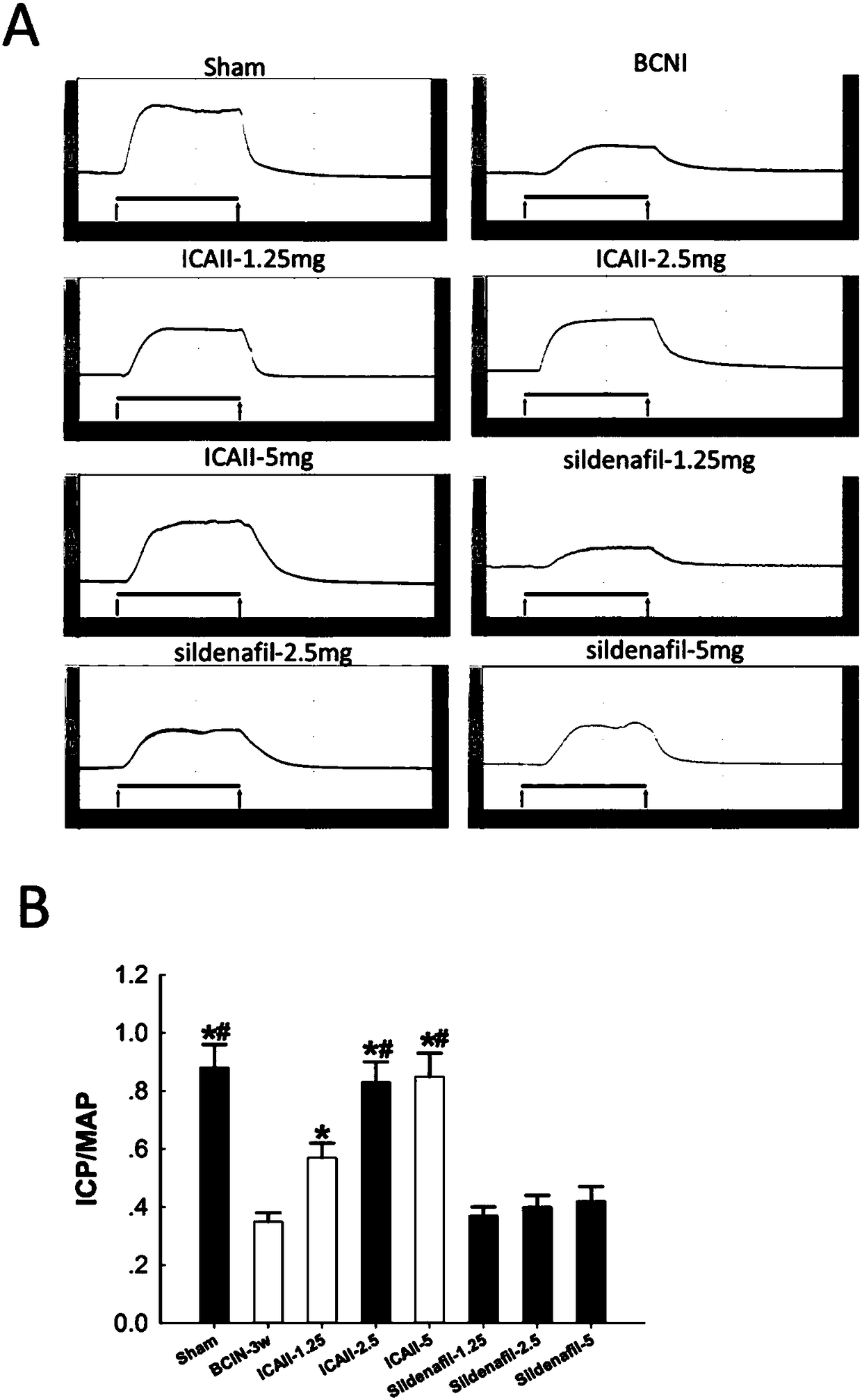
[0056] After 12 weeks, 12 rats were randomly selected as the sham operation group (Sham, laparotomy without injury to the cavernous nerve, n=12). The remaining 84 SD rats were injured bilateral cavernous nerves (see 2) to establish a neurogenic ED model; randomly selected 12 rats were fed with sildenafil, 1.25mg / kg / d; randomly selected 12 rats were fed with sildenafil Nafil, 2.5mg / kg / d; 12 randomly selected animals were fed with sildenafil, 5mg / kg / d; 12 randomly selected dogs were fed with icariside Ⅱ, 2.5mg / kg / d; randomly selected 12 rats were fed with icariin Ⅱ, 2.5 mg / kg / d; 12 rats were randomly selected and fed with icariin Ⅱ, 5 mg / kg / d; the remaining 12 rats were used as the model group (BCNI-3w).
[0057] (2) Establishment of neurogenic ED rat model:
[0058] After SD rats were born for 12 weeks, the ED animal model was established by inj...
Embodiment 2
[0077] Penile Erectile Function Assessment
[0078] Such as figure 1 Shown, daily feeding of sildenafil does not significantly improve the erectile function of the neurogenic ED rat model (VSBCNI-3w group); feeding ICAⅡ1.25mg / kg, 2.5mg / kg and 5mg / kg, It can significantly improve the erectile function of the neurogenic ED rat model (VSBCNI-3w group); and the ICAⅡ2.5mg / kg and 5mg / kg groups have significant differences compared with the ICAⅡ1.25mg / kg group; and the ICAⅡ2.5mg / kg kg and 5mg / kg group, ICAⅡ 5mg / kg group did not improve the penile erectile energy of rats significantly.
Embodiment 3
[0080] experimental method:
[0081] (1) Labeling systemic endogenous stem cells / precursor cells using the technique of labeling resident cells
[0082] Dissolve 50mg of EdU in 2.5ml of DMSO, add 2.5ml of sterilized deionized water, mix well, and keep warm in a 37-degree water bath. Within 12 hours after birth, the suckling mice were intraperitoneally injected with 50 μl of EdU solution at a dosage of 50 mg / kg.
[0083] (2) Experimental animals and grouping
[0084] Forty-eight newborn 1-day-old male SD rats were intraperitoneally injected with EdU-labeled endogenous stem / precursor cells.
[0085] After 12 weeks, 12 rats were randomly selected as the sham operation group (Sham, laparotomy without injury to the cavernous nerve, n=12). The remaining 48 SD rats were injured bilateral cavernous nerves (see 4) to establish neurogenic ED models; randomly selected 12 rats were fed with sildenafil, 2.5mg / kg / d; the remaining 12 rats were fed with epimedium Hyposide Ⅱ, 2.5mg / kg / d). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com