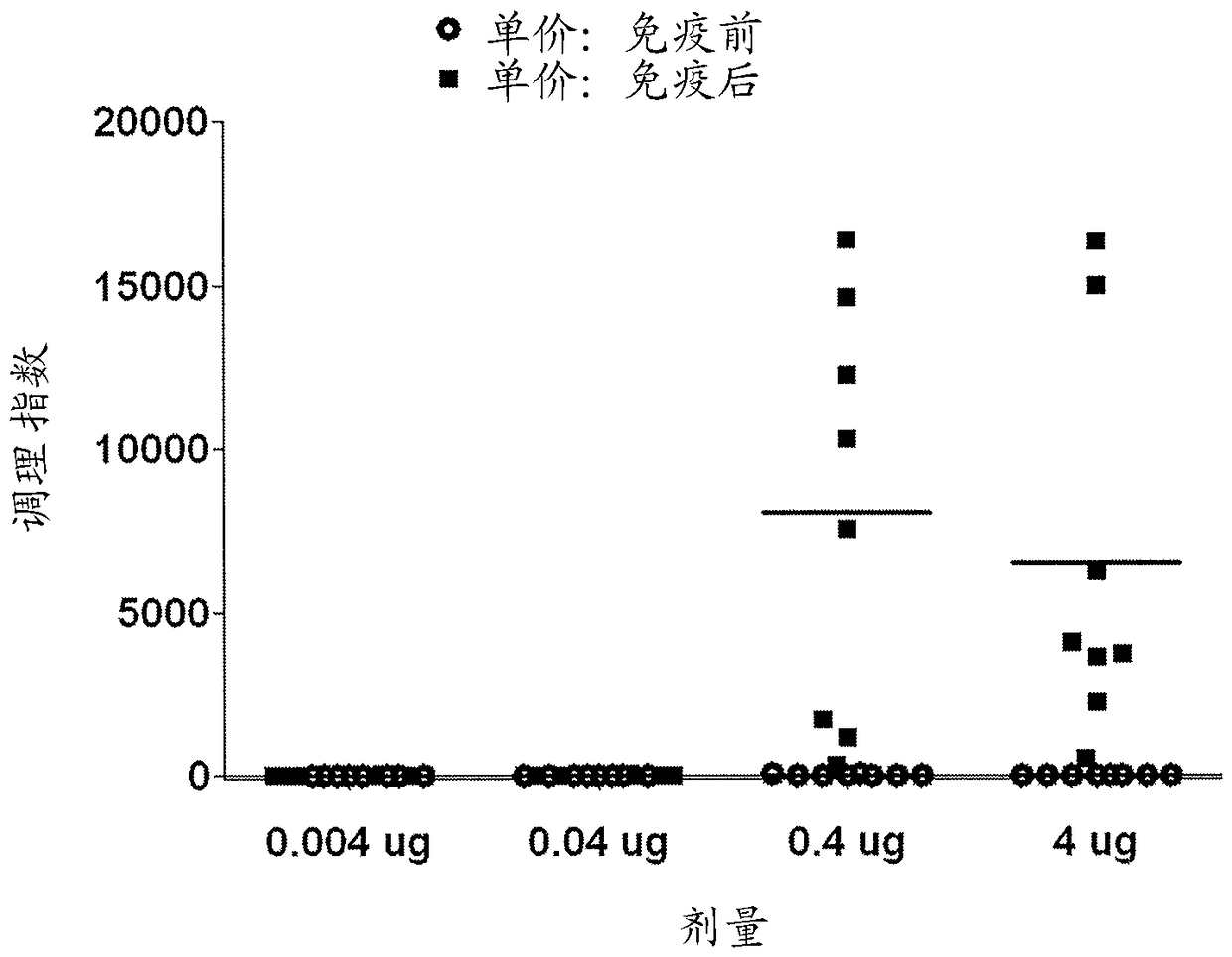
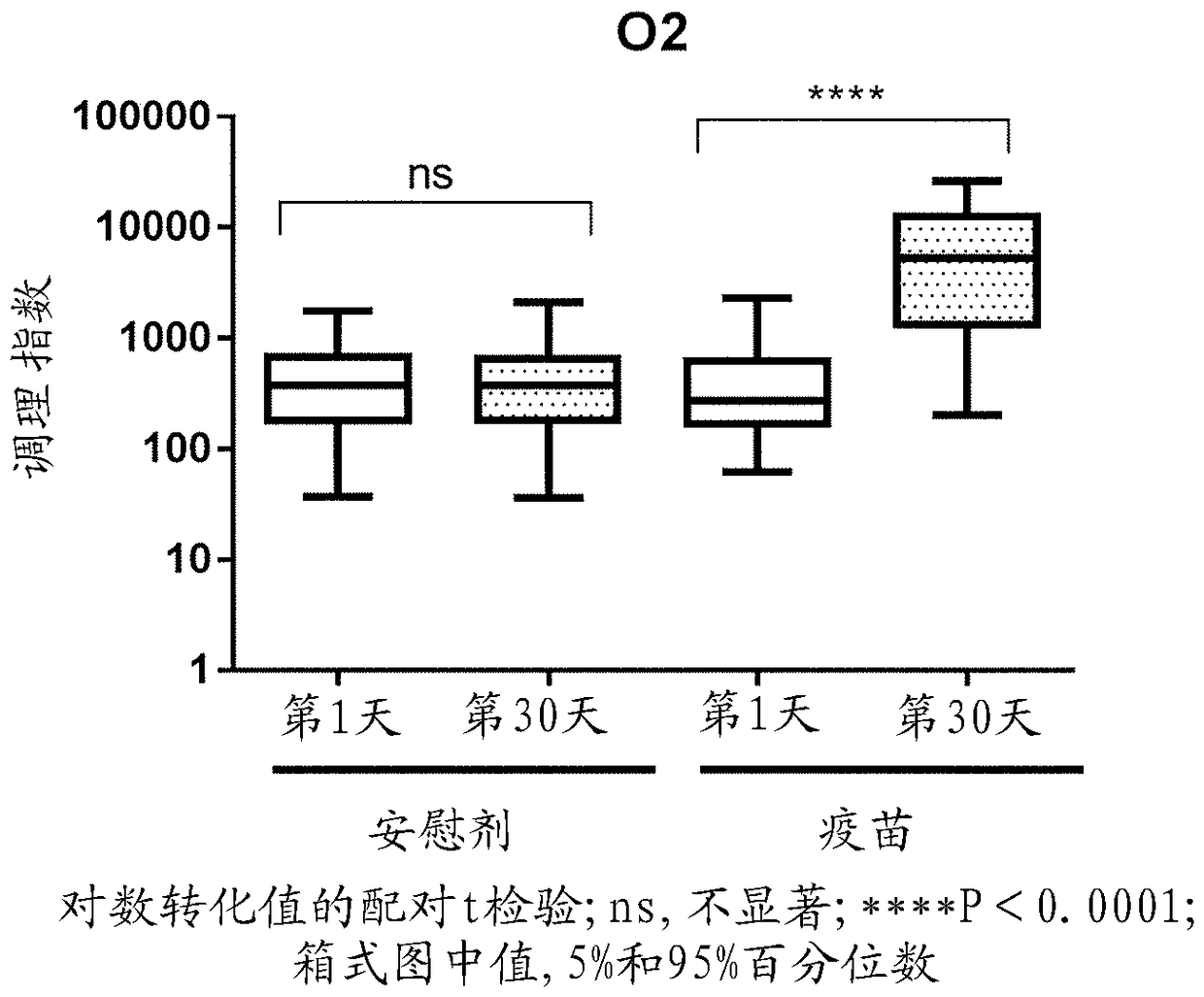
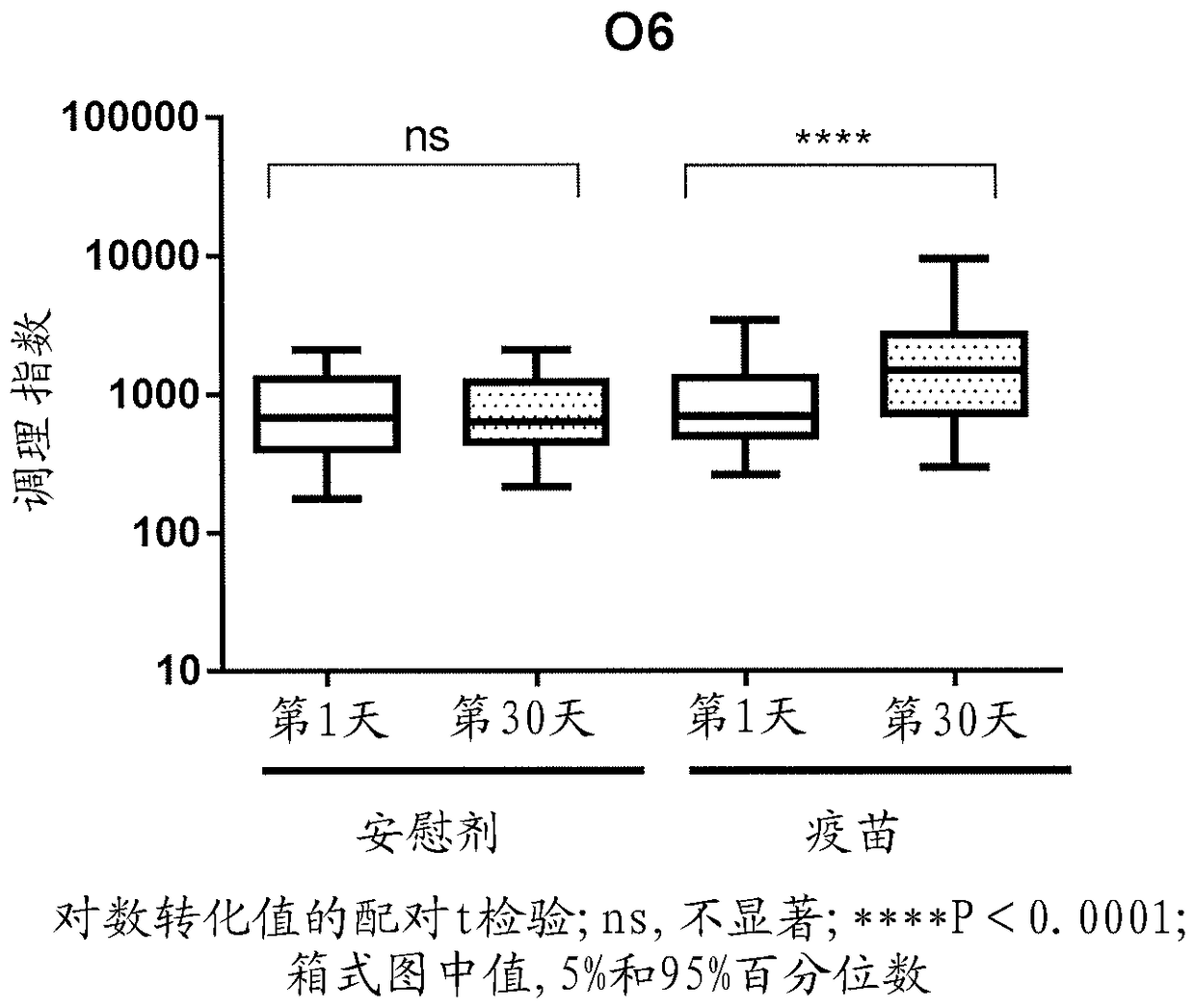
Methods and compositions for immune protection against extra-intestinal pathogenic E. coli
A technology of Escherichia coli and composition, applied in the field of immune protection and composition against extraintestinal pathogenic Escherichia coli, and can solve the problems of increasing serotype carrying and disease, weak efficacy, weak immunogenicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0180] Embodiment 1 is a composition comprising a first concentration of Escherichia coli O25B antigen polysaccharide, and a second concentration of Escherichia coli O1A antigen polysaccharide, Escherichia coli O2 antigen polysaccharide and Escherichia coli O6A antigen polysaccharide, wherein the first concentration and the second The ratio of concentration is 1:1 to 2:1, Escherichia coli O25B, O1A, O2 and O6A antigenic polysaccharides are each independently covalently bound to detoxified exotoxin A (EPA) of Pseudomonas aeruginosa carrier protein, and the first concentration From 10 to 36 μg / ml.
Embodiment approach 2
[0181] Embodiment 2 is the composition of embodiment 1 comprising Escherichia coli O25B, O1A, O2, and O6A antigenic polysaccharides in a weight ratio of 1:1:1:1.
Embodiment approach 3
[0182] Embodiment 3 is the composition of embodiment 1 comprising the O25B, O1A, O2, and O6A antigenic polysaccharides in a weight ratio of 2:1:1:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com