Bivalent bromodomain inhibitors and uses thereof
A compound and pharmaceutical technology, applied in the direction of anti-toxic agents, anti-inflammatory agents, non-central analgesics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0768] These and other aspects of the invention will be further appreciated upon consideration of the following examples, which are intended to illustrate some specific embodiments of the invention, but are not intended to limit its scope as defined in the claims.
[0769] Potent and selective bivalent inhibitors of BET family bromodomains
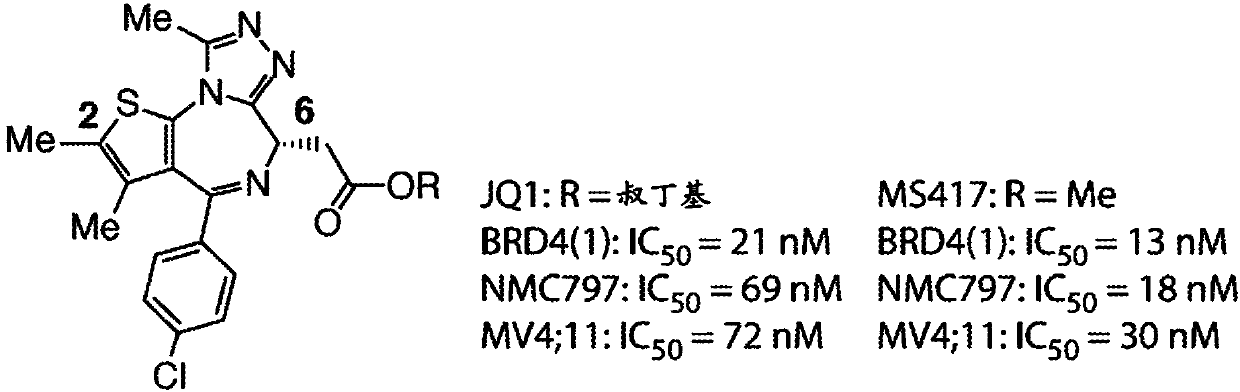
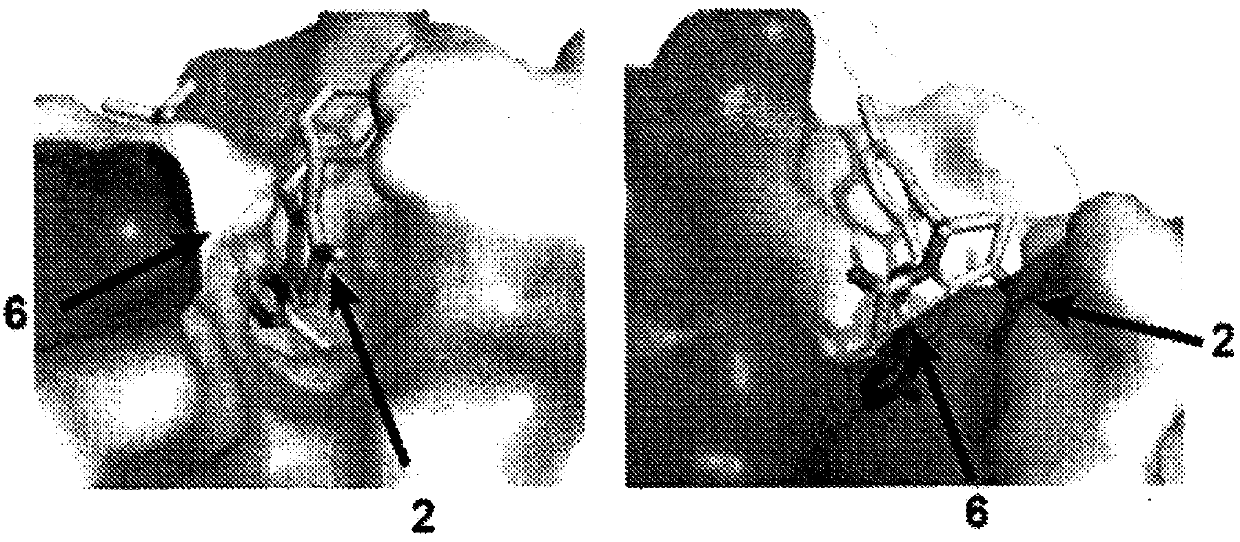
[0770] Design, preparation and evaluation of bivalent inhibitors of BRD4. Previous high-resolution structures of JQ1 bound to BD1 and BD2 (see, e.g., Filippakopoulos, P. et al. Nature 2010, 468, 1067-1073) and internal structure-activity relationship (SAR) guidance support the diazepine The chemical substitution of the bulky tert-butyl ester functional group at C6 on the alkene ring and the substitution of the methyl moiety at C2 on the thiophene ring, since both are localized to the solvent ( Figure 1b ). Since the BD1 and BD2 bromodomains are separated by a 280-residue linking region ( Figure 1c ), and since binding can be intramole...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com