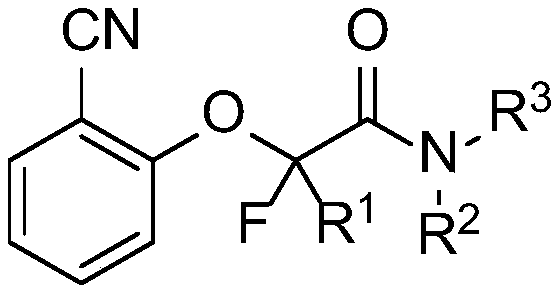
A kind of cyanofluoroamide compound with antibacterial activity and its preparation method and application
A technology of cyanofluoramide and antibacterial activity, which is applied in the field of chemical synthesis of drugs, can solve the problems of decreased efficacy of fungicides, and achieve excellent bactericidal and antibacterial effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
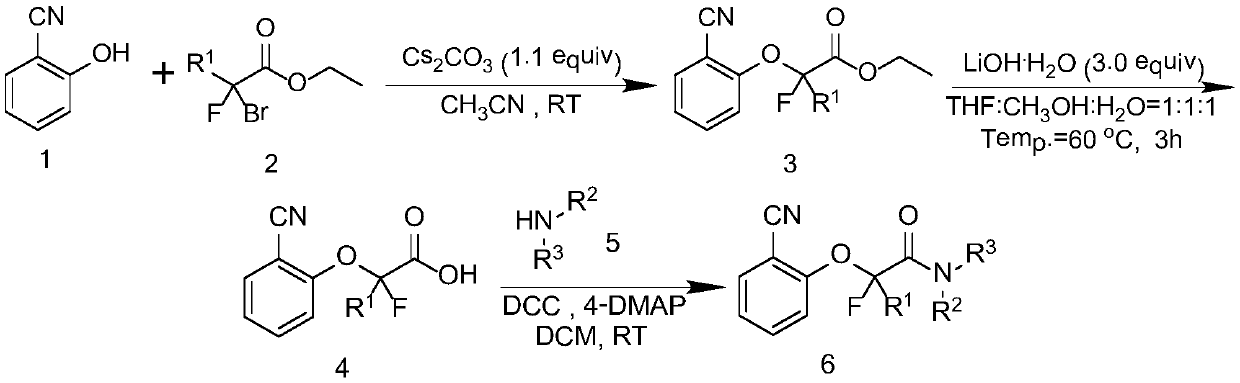
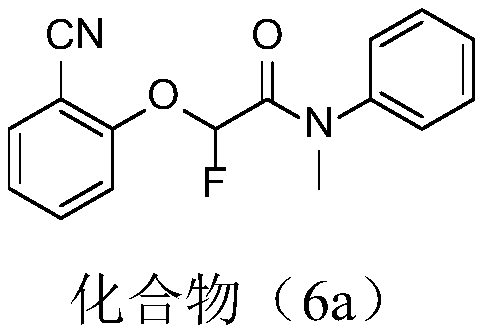
[0030] Embodiment 1: the synthesis of compound 2-(2-cyanophenoxy)-2-fluoro-N-methyl-N-phenylacetamide (6a)
[0031]
[0032] 2-(2-cyanophenoxy)-2-fluoroacetic acid was prepared by referring to the method disclosed in literature (Nature, 2014, 507, 215–220.).
[0033] 2-(2-cyanophenoxy)-2-fluoroacetic acid (compound 4), N-methylaniline, DCC (dicyclohexylcarbodiimide) and 4-DMAP (4-dimethylaminopyridine) , according to the molar ratio of 1:1.2:1.1:0.2 into the reaction flask, the amount of compound 4 is 1.5mmol. The reaction system was stirred and reacted in DCM solvent (dichloromethane) at room temperature for 16 hours. After the reaction, cool down, filter under reduced pressure with a short silica gel column, and rotate the filtrate to remove the solvent. The residue is chromatographed on a silica gel column, rinsed with PE:EtOAc=1:1 developer, detected by TLC, and the effluents containing the product are combined. solution, the solvent was distilled off by a rotary evap...
Embodiment 2
[0036] Example 2: Synthesis of compound 2-(2-cyanophenoxy)-2-fluoro-N-phenylacetamide (6b).
[0037] The synthesis method was the same as in Example 1, and 2-(2-cyanophenoxy)-2-fluoroacetic acid was reacted with aniline to obtain light yellow solid compound (6b) with a yield of 86%.
[0038]
[0039] Compound (6b) NMR analysis data are as follows:
[0040] 1 H NMR (600MHz, CDCl 3 )δ8.56(s,1H),7.78–7.56(m,4H),7.42–7.33(m,3H),7.30(t,J=7.6Hz,1H),7.19(t,J=7.4Hz,1H ),6.08(d,J=59.0Hz,1H). 13 C NMR (151MHz, CDCl 3 )δ160.4(d,J C-F =25.5Hz,1C),156.7(d,J C-F =2.7Hz,1C),136.3,135.0,133.6,129.2,125.5,125.0,120.1,116.2,115.7,104.1,103.1(d,J C-F =236.6Hz,1C).
Embodiment 3
[0041] Example 3: Synthesis of compound N-(2-bromophenyl)-2-(2-cyanophenoxy)-2-fluoroacetamide (6c).
[0042] The synthesis method was the same as that in Example 1, and 2-(2-cyanophenoxy)-2-fluoroacetic acid was reacted with 2-bromoaniline to obtain light yellow solid compound (6c), with a yield of 63%.
[0043]
[0044] Compound (6c) NMR analysis data are as follows:
[0045] 1 H NMR (600MHz, CDCl 3 )δ8.89(s,1H),8.37(dd,J=8.2,1.4Hz,1H),7.71–7.63(m,2H),7.59(dd,J=8.0,1.3Hz,1H),7.39–7.32 (m,2H),7.29(t,J=7.6Hz,1H),7.07(td,J=7.9,1.5Hz,1H),6.18(d,J=59.0Hz,1H). 13 C NMR (151MHz, CDCl 3 )δ160.5(d,J C-F =25.3Hz,1C),156.1(d,J C-F =2.6Hz,1C),134.8,134.2,134.1,132.6,128.4,126.5,124.9,122.1,116.0,115.1,114.4,104.2,103.0(d,J C-F =237.3Hz,1C).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap