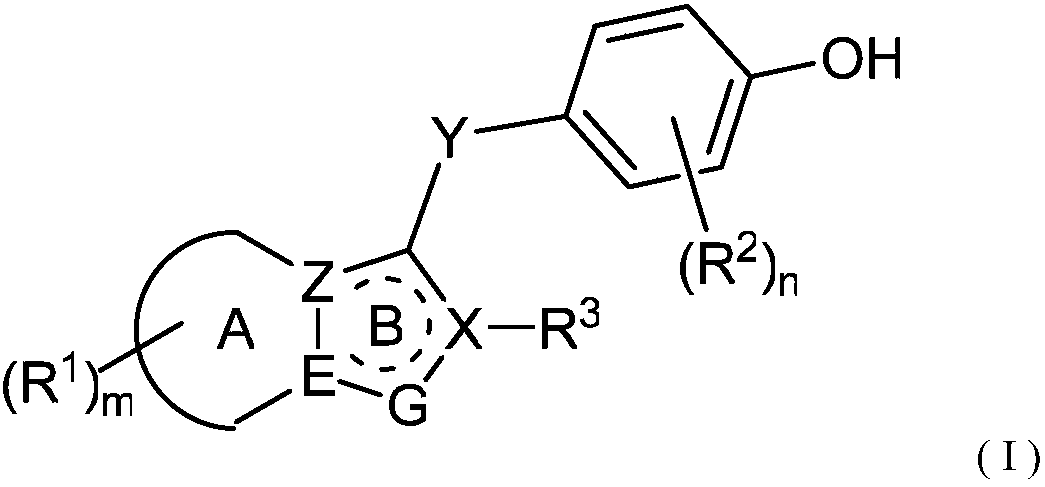
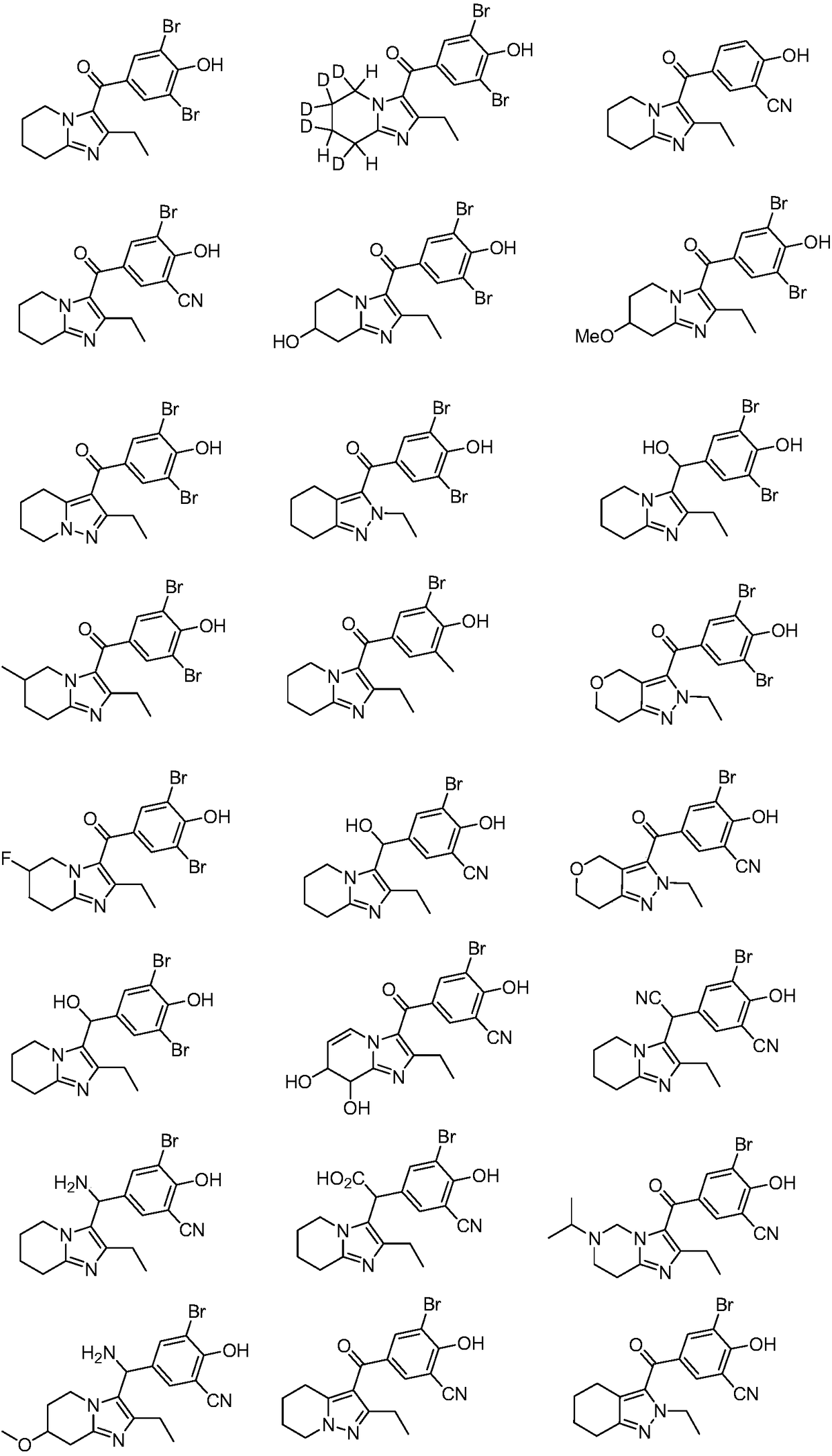
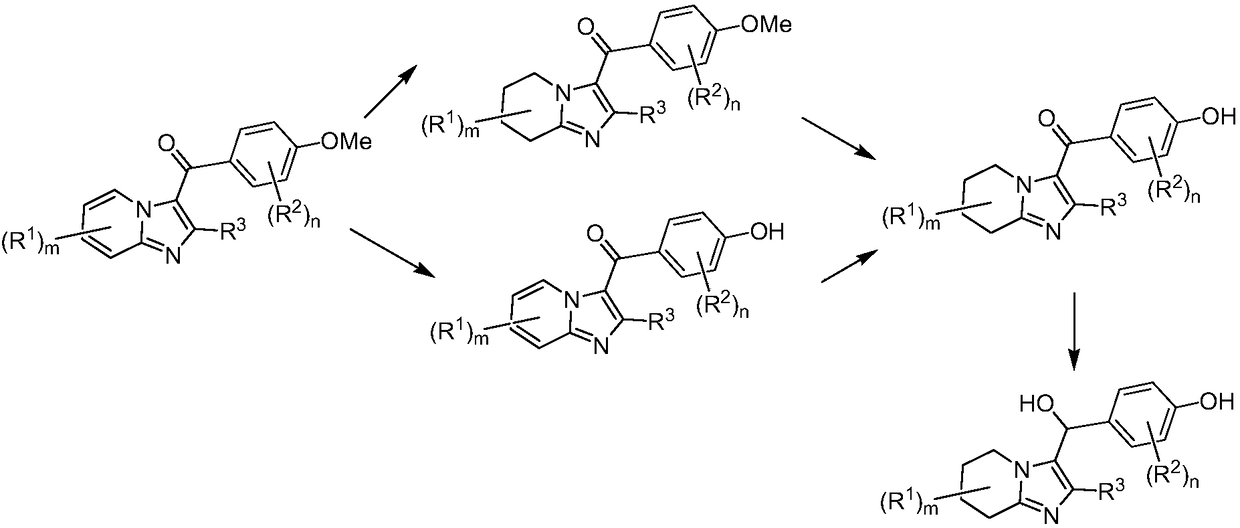
URAT1 inhibitor for promoting uric acid excretion
A compound and pharmaceutical technology, applied in the field of URAT1 inhibitor compounds, can solve the problems of high incidence of serious adverse events, bleak future, and lack of significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: (3,5-dibromo-4-hydroxyphenyl)(2-ethyl-5,6,7,8-tetrahydroimidazo[1,2-a]pyridin-3-yl)methanol Synthesis of Ketone (5)
[0072]
[0073] Step A: Dissolve 2-aminopyridine (2.0g, 21.3mmol) and triethylamine (2.58g, 25.5mmol) in dichloromethane (20mL), then add propionyl chloride (2.07g, 22.4mmol) dropwise in an ice-water bath ). After the addition was complete, the resulting mixture was stirred overnight at room temperature. Water (40 mL) was added, extracted with dichloromethane (40 mL×3), the combined organic phases were washed with saturated brine (30 mL), and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the product was purified by column chromatography (200-300 mesh silica gel, ethyl acetate:petroleum ether=1:15-1:10 elution) to obtain N-(pyridin-2-yl)propionamide ( 1) (2.74g). The yield was 85.6%.
[0074] Step B: A mixture containing compound 1 (300 mg, 2.0 mmol), 2-bromo-1-(4-methoxyphenyl)ethanone (460...
Embodiment 2
[0078] Example 2: (3,5-dibromo-4-hydroxyphenyl)(5,6,6,7,8-pentadeutero-2-ethyl-5,6,7,8-tetrahydroimidazo[ Synthesis of 1,2-a]pyridin-3-yl)methanone (10)
[0079]
[0080] Step A: Add 60% sodium hydride (1.68 g, 42 mmol) in portions to a solution of p-methoxyacetophenone (3.0 g, 20.0 mmol) in DMF (15 mL) at -10-0°C. After the addition was complete, stirring was continued at this temperature for 40 minutes, then ethyl propionate (2.04 g, 20 mmol) was added dropwise. After the addition was complete, the resulting mixture was stirred overnight at room temperature. Water (60 mL) was added, extracted with ethyl acetate (30 mL×3), the combined organic phases were washed with saturated brine (20 mL×2), and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the product was purified by column chromatography (200-300 mesh silica gel, ethyl acetate:petroleum ether=1:30 elution) to obtain 1-(4-methoxyphenyl)pentane-1.3- Diketone (6) (3.16 g). ...
Embodiment 3
[0084] Example 3: Synthesis of 5-(2-ethyl-5,6,7,8-tetrahydroimidazo[1,2-a]pyridine-3-carbonyl)-2-hydroxybenzonitrile (16)
[0085]
[0086] Step A: Add 4-methoxyacetophenone (44.0 g, 293 mmol) to 1-chloromethyl-4-fluoro-1,4-diazabicyclo[2.2.2]octane alkane di(tetrafluoroborate) salt (104g, 294mmol), iodine (38.6g, 152mmol) and acetonitrile (440mL). After the addition was complete, the resulting mixture was stirred overnight at room temperature. Water (1350 mL) was added to the reaction mixture, and a large amount of solid precipitated out. Filtration and drying gave 3-iodo-4-methoxyacetophenone (11) (70.0 g). The yield was 86.5%.
[0087] Step B: A mixture containing compound 11 (70.0 g, 254 mmol), cuprous cyanide (34.0 g, 380 mmol) and DMF (400 mL) was stirred at 130 °C overnight. Cool to room temperature, filter through diatomaceous earth, add water (1600mL), extract with ethyl acetate (800mL×3), the combined organic phases are washed with water (400mL×2) and saturate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com